Polyvascular Disease and Risk of Major Adverse Cardiovascular Events in Peripheral Artery Disease: A Secondary Analysis of the EUCLID Trial
- PMID: 30646395
- PMCID: PMC6324381
- DOI: 10.1001/jamanetworkopen.2018.5239
Polyvascular Disease and Risk of Major Adverse Cardiovascular Events in Peripheral Artery Disease: A Secondary Analysis of the EUCLID Trial
Abstract
Importance: The effect of polyvascular disease on cardiovascular outcomes in the background of peripheral artery disease (PAD) is unclear.
Objective: To determine the risk of ischemic events (both cardiac and limb) among patients with PAD and polyvascular disease.
Design, setting, and participants: In this post hoc secondary analysis of the international Examining Use of Ticagrelor in Peripheral Artery Disease (EUCLID) trial, outcomes were compared among 13 885 enrolled patients with PAD alone, PAD + coronary artery disease (CAD), PAD + cerebrovascular disease (CVD), and PAD + CAD + CVD. Adjusted Cox proportional hazards regression models were implemented to determine the risk associated with polyvascular disease and outcomes, and intention-to-treat analysis was performed. The EUCLID trial was conducted from December 31, 2012, to March 7, 2014; the present post hoc analysis was performed from June 1, 2017, to February 5, 2018.
Interventions: EUCLID evaluated ticagrelor vs clopidogrel in preventing major adverse cardiac events (cardiovascular death, myocardial infarction [MI], or ischemic stroke) and major bleeding in patients with PAD.
Main outcomes and measures: The primary end point was a composite of cardiovascular death, MI, or ischemic stroke. Key secondary end points included the individual components of the primary end point and acute limb ischemia leading to hospitalization, major amputation, and lower-extremity revascularization. The primary end point of Thrombolysis in Myocardial Infarction (TIMI) major bleeding was also evaluated.
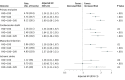
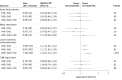
Results: The EUCLID trial randomized 13 885 patients with a median age of 66 years (interquartile range, 60-73 years), of whom 3888 (28.0%) were women. At baseline, 7804 patients (56.2%) had PAD alone; 2639 (19.0%) had PAD + CAD; 2049 (14.8%) had PAD + CVD; and 1393 (10.0%) had PAD + CAD + CVD. Compared with patients with isolated PAD, the adjusted hazard ratios (aHRs) for major adverse cardiac events were 1.34 (95% CI, 1.15-1.57; P < .001) for PAD + CVD, 1.65 (95% CI, 1.43-1.91; P < .001) for PAD + CAD, and 1.99 (95% CI, 1.69-2.34; P < .001) for PAD + CAD + CVD. The aHRs for lower-extremity revascularization were 1.17 (95% CI, 1.03-1.34; P = .01) for PAD + CAD, 1.17 (95% CI, 1.02-1.35; P = .02) for PAD + CVD, and 1.34 (95% CI, 1.15-1.57; P < .001) for PAD + CAD + CVD. Polyvascular disease was not associated with an increased risk of acute limb ischemia (aHR for PAD + CVD, 0.91; 95% CI, 0.62-1.34, P = .63; PAD + CAD, 0.93; 95% CI, 0.64-1.34, P = .69; and PAD + CAD + CVD, 0.98; 95% CI, 0.63-1.53, P = .93), major amputation (aHR for PAD + CVD, 0.83; 95% CI, 0.54-1.27, P = .40; PAD + CAD, 0.74; 95% CI, 0.47-1.16, P = .19; and PAD + CAD + CVD, 1.12; 95% CI, 0.69-1.80, P = .65), or TIMI major bleeding (PAD + CVD, 0.98; 0.66-1.44, P = .91; PAD + CAD, 1.04; 0.74-1.48, P = .81; and PAD + CAD + CVD, 0.96; 95% CI, 0.62-1.51, P = .88).
Conclusions and relevance: Compared with patients with PAD alone, the risk of major adverse cardiac events and lower-extremity revascularization increased with multiple vascular bed involvement. There was no clear increased risk of bleeding associated with polyvascular disease.
Conflict of interest statement
Figures

References
-
- Bonaca MP, Gutierrez JA, Creager MA, et al. . Acute limb ischemia and outcomes with vorapaxar in patients with peripheral artery disease: results from the Trial to Assess the Effects of Vorapaxar in Preventing Heart Attack and Stroke in Patients With Atherosclerosis-Thrombolysis in Myocardial Infarction 50 (TRA2°P-TIMI 50). Circulation. 2016;133(10):997-1005. doi:10.1161/CIRCULATIONAHA.115.019355 - DOI - PubMed
-
- Gerhard-Herman MD, Gornik HL, Barrett C, et al. . 2016 AHA/ACC guideline on the management of patients with lower extremity peripheral artery disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2017;135(12):e726-e779. doi:10.1161/CIR.0000000000000471 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

