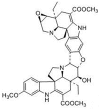
Pharmacokinetics, Tissue Distribution, Plasma Protein Binding Studies of 10-Dehydroxyl-12-Demethoxy-Conophylline, a Novel Anti-Tumor Candidate, in Rats
- PMID: 30646543
- PMCID: PMC6359039
- DOI: 10.3390/molecules24020283
Pharmacokinetics, Tissue Distribution, Plasma Protein Binding Studies of 10-Dehydroxyl-12-Demethoxy-Conophylline, a Novel Anti-Tumor Candidate, in Rats
Abstract
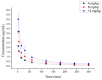
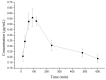
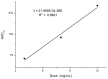
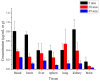
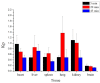
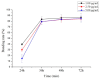
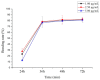
10-Dehydroxyl-12-demethoxy-conophylline is a natural anticancer candidate. The motivation of this study was to explore the pharmacokinetic profiles, tissue distribution, and plasma protein binding of 10-dehydroxyl-12-demethoxy-conophylline in Sprague Dawley rats. A rapid, sensitive, and specific ultra-performance liquid chromatography (UPLC) system with a fluorescence (FLR) detection method was developed for the determination of 10-dehydroxyl-12-demethoxy-conophylline in different rat biological samples. After intravenous (i.v.) dosing of 10-dehydroxyl-12-demethoxy-conophylline at different levels (4, 8, and 12 mg/kg), the half-life t1/2α of intravenous administration was about 7 min and the t1/2β was about 68 min. The AUC0→∞ increased in a dose-proportional manner from 68.478 μg/L·min for 4 mg/kg to 305.616 mg/L·min for 12 mg/kg. After intragastrical (i.g.) dosing of 20 mg/kg, plasma levels of 10-dehydroxyl-12-demethoxy-conophylline peaked at about 90 min. 10-dehydroxyl-12-demethoxy-conophyllinea absolute oral bioavailability was only 15.79%. The pharmacokinetics process of the drug was fit to a two-room model. Following a single i.v. dose (8 mg/kg), 10-dehydroxyl-12-demethoxy-conophylline was detected in all examined tissues with the highest in kidney, liver, and lung. Equilibrium dialysis was used to evaluate plasma protein binding of 10-dehydroxyl-12-demethoxy-conophylline at three concentrations (1.00, 2.50, and 5.00 µg/mL). Results indicated a very high protein binding degree (over 80%), reducing substantially the free fraction of the compound.
Keywords: 10-dehydroxyl-12-demethoxy-conophylline; pharmacokinetics; protein binding rate; tissue distribution.
Conflict of interest statement
The authors declare no conflict of interest
Figures
Similar articles
-
Pharmacokinetics, tissue distribution, excretion and plasma protein binding studies of wogonin in rats.Molecules. 2014 Apr 29;19(5):5538-49. doi: 10.3390/molecules19055538. Molecules. 2014. PMID: 24786691 Free PMC article.
-
Pharmacokinetics, Tissue Distribution and Protein Binding Studies of Chrysocauloflavone I in Rats.Planta Med. 2016 Feb;82(3):217-23. doi: 10.1055/s-0035-1558159. Epub 2015 Nov 17. Planta Med. 2016. PMID: 26576031
-
Pharmacokinetics, tissue distribution and excretion of vinflunine.Eur J Drug Metab Pharmacokinet. 2006 Apr-Jun;31(2):59-64. doi: 10.1007/BF03191120. Eur J Drug Metab Pharmacokinet. 2006. PMID: 16898072
-
Conophylline: a novel differentiation inducer for pancreatic beta cells.Int J Biochem Cell Biol. 2006;38(5-6):923-30. doi: 10.1016/j.biocel.2005.09.019. Epub 2005 Oct 26. Int J Biochem Cell Biol. 2006. PMID: 16337165 Review.
-
Therapeutic activity of plant-derived alkaloid conophylline on metabolic syndrome and neurodegenerative disease models.Hum Cell. 2018 Apr;31(2):95-101. doi: 10.1007/s13577-017-0196-4. Epub 2017 Dec 16. Hum Cell. 2018. PMID: 29249016 Review.
Cited by
-
The Influence of Palmatine Isolated from Berberis sibiricaRadix on Pentylenetetrazole-Induced Seizures in Zebrafish.Cells. 2020 May 16;9(5):1233. doi: 10.3390/cells9051233. Cells. 2020. PMID: 32429356 Free PMC article.
-
Comparison of Pinoresinol and its Diglucoside on their ADME Properties and Vasorelaxant Effects on Phenylephrine-Induced Model.Front Pharmacol. 2021 Aug 9;12:695530. doi: 10.3389/fphar.2021.695530. eCollection 2021. Front Pharmacol. 2021. PMID: 34434107 Free PMC article.
References
-
- Qu Y., Easson M., Simionescu R., Hajicek J., Thamm A.M.K., Salim V., De Luca V. Solution of the multistep pathway for assembly of corynanthean, strychnos, iboga, and aspidosperma monoterpenoid indole alkaloids from 19E-geissoschizine. Proc. Natl Acad. Sci. USA. 2018;115:3180–3185. doi: 10.1073/pnas.1719979115. - DOI - PMC - PubMed
-
- Kumar S.R., Shilpashree H.B., Nagegowda D.A. Terpene moiety enhancement by overexpression of geranyl(geranyl) diphosphate synthase and geraniol synthase elevates monomeric and dimeric monoterpene indole alkaloids in transgenic catharanthus roseus. Front. Plant Sci. 2018;9:942. doi: 10.3389/fpls.2018.00942. - DOI - PMC - PubMed
-
- Coatti G.C., Marcarini J.C., Sartori D., Fidelis Q.C., Ferreira D.T., Mantovani M.S. Cytotoxicity, genotoxicity and mechanism of action (via gene expression analysis) of the indole alkaloid aspidospermine (antiparasitic) extracted from Aspidosperma polyneuron in HepG2 cells. Cytotechnology. 2016;68:1161–1170. doi: 10.1007/s10616-015-9874-9. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

