A Randomized Controlled, Treat-to-Target Study Evaluating the Efficacy and Safety of Insulin Glargine 300 U/mL (Gla-300) Administered Using Either Device-Supported or Routine Titration in People With Type 2 Diabetes
- PMID: 30646755
- PMCID: PMC6955447
- DOI: 10.1177/1932296818821706
A Randomized Controlled, Treat-to-Target Study Evaluating the Efficacy and Safety of Insulin Glargine 300 U/mL (Gla-300) Administered Using Either Device-Supported or Routine Titration in People With Type 2 Diabetes
Abstract
Background: The efficacy/safety of device-supported versus routine titration with Gla-300 in type 2 diabetes (T2DM) was evaluated.
Method: AUTOMATIX was a 16-week, randomized, open-label, parallel-group, multicenter, noninferiority trial in insulin-treated or insulin-naïve people with T2DM. The fasting self-monitored plasma glucose (FSMPG) target was 90-130 mg/dL (5.0-7.2 mmol/L). Primary endpoint: proportion of participants achieving target FSMPG at week 16 without severe hypoglycemia. Secondary endpoints included: proportion reaching FSMPG target without confirmed (≤70 mg/dL [≤3.9 mmol/L]) or severe hypoglycemia; time to first achieve FSMPG target; mean FSMPG and HbA1c change (baseline to week 16). Safety endpoints included hypoglycemia and adverse events. Patient-reported outcomes (PROs) were also assessed.
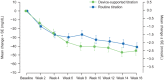
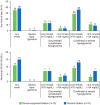
Results: Participants were randomized to device-supported (n = 75) or routine titration (n = 76); 17 participants in the device-supported group discontinued device use. Noninferiority was achieved for the primary endpoint (device-supported: 45.9%, routine: 36.8%; weighted difference: 9.04 [95% CI: -6.75, 24.83]), but not superiority (P = .262). The proportion reaching FSMPG target range without confirmed (≤70 mg/dL [≤3.9 mmol/L]) or severe hypoglycemia was 34.3% vs 14.5%, respectively. The time at which 50% of the participants achieved the FSMPG target was less in the device-supported than routine titration arm (10 vs 13 weeks). Least squares mean HbA1c reduction, safety profiles, and PROs were similar in both arms. Mean "ease of use" score for the device, assessed by healthcare professionals and participants on a scale of 1-7, was ≥6.
Conclusions: Device-supported self-titration had a good safety/efficacy profile, and was noninferior to routine titration and well accepted by diabetes specialists and patients.
Keywords: basal insulin; blood glucose meter; hypoglycemia; titration.
Conflict of interest statement
Figures

References
-
- Nelson SE, Palumbo PJ. Addition of insulin to oral therapy in patients with type 2 diabetes. Am J Med Sci. 2006;331:257-263. - PubMed
-
- American Diabetes Association. Standards of medical care in diabetes—2017. Diabetes Care. 2017;39:S1-S135.
-
- Wu N, Aagren M, Boulanger L, Friedman M, Wilkey K. Assessing achievement and maintenance of glycemic control by patients initiating basal insulin. Curr Med Res Opin. 2012;28:1647-1656. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

