Programmed cell death ligand 1 (PD-L1, CD274) in cholangiocarcinoma - correlation with clinicopathological data and comparison of antibodies
- PMID: 30646854
- PMCID: PMC6332835
- DOI: 10.1186/s12885-018-5254-0
Programmed cell death ligand 1 (PD-L1, CD274) in cholangiocarcinoma - correlation with clinicopathological data and comparison of antibodies
Abstract
Background: Cholangiocarcinoma (CCA) may arise in the intra- or extrahepatic biliary tract and is associated with a poor prognosis. Despite recent advances, to date there is still no established targeted therapeutic approach available. Non-surgical therapeutic agents are urgently needed, as most patients are non-eligible to surgical resection. Anti-PD-L1 therapy prevents cancer cells from evading the immune system and has emerged as a new treatment option in several cancer entities. Recently, PD-L1 expression has been analyzed in comparably small CCA patient cohorts. However, a systematic validation of different PD-L1 antibodies has not been performed in CCA so far.
Methods: We stained a tissue microarray consisting of 170 patients, including 72 intrahepatic cholangiocarcinomas (iCCAs), 57 perihilar cholangiocarcinomas (pCCAs) and 41 distal cholangiocarcinomas (dCCAs) by immunohistochemistry and evaluated PD-L1 positivity in tumor and stromal cells. We analyzed three different PD-L1 antibodies (clones 28-8, SP142, and SP263) that are frequently used and recommended for predictive diagnostic testing in other cancer types.
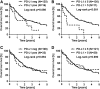
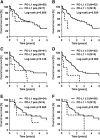
Results: For PD-L1 antibody clone SP263, 5% of iCCAs, 4% of pCCAs and 3% of dCCAs exhibited PD-L1 expression on tumor cells, thereby showing the highest frequencies of PD-L1 positivity. Accordingly, highest PD-L1 positivity rates of stromal cells with 31% in iCCA, 40% in pCCA and 61% in dCCA were detected for clone SP263. Agreement of PD-L1 positivity in tumor cells was moderate for clone 28-8 and SP263 (κ = 0.44) and poor between 28-8 and SP142 (κ = 0.13), as well as SP142 and SP263 (κ = 0.11), respectively. Statistical analyses of PD-L1 expression (clone SP263) on tumor cells with clinicopathological data revealed a positive correlation with shortened overall survival in CCA patients.
Conclusions: Selection of appropriate PD-L1 antibodies and careful evaluation of immunohistochemical staining patterns have a significant impact on PD-L1 testing in CCA. Clinical trials are necessary to investigate the putative beneficial effects of PD-L1 targeted immunotherapy in CCA patients.
Keywords: 28–8; CD274; Cholangiocarcinoma; PD-L1; SP142; SP263.
Conflict of interest statement
Ethics approval and consent to participate
Tissues were used in accordance with the ethical regulations of the NCT tissue bank established by the local ethics committee (S-207/2015). Ethics approval, written and verbal consent to participate has been given by all participants.
Consent for publication
Consent for publication has been obtained (NCT; project # 2116 and S-207/2015).
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

References
-
- Daud AI, Wolchok JD, Robert C, Hwu WJ, Weber JS, Ribas A, Hodi FS, Joshua AM, Kefford R, Hersey P, et al. Programmed death-ligand 1 expression and response to the anti-programmed death 1 antibody Pembrolizumab in melanoma. J Clin Oncol. 2016;34(34):4102–4109. doi: 10.1200/JCO.2016.67.2477. - DOI - PMC - PubMed
-
- Shukuya T, Mori K, Amann JM, Bertino EM, Otterson GA, Shields PG, Morita S, Carbone DP. Relationship between overall survival and response or progression-free survival in advanced non-small cell lung Cancer patients treated with anti-PD-1/PD-L1 antibodies. J Thorac Oncol. 2016;11(11):1927–1939. doi: 10.1016/j.jtho.2016.07.017. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

