Absence of early metabolic response assessed by 18F-FDG PET/CT after initiation of antifibrotic drugs in IPF patients
- PMID: 30646908
- PMCID: PMC6334423
- DOI: 10.1186/s12931-019-0974-5
Absence of early metabolic response assessed by 18F-FDG PET/CT after initiation of antifibrotic drugs in IPF patients
Abstract
Background: Idiopathic pulmonary fibrosis (IPF) is characterized by a progressive and irreversible respiratory failure. Non-invasive markers of disease activity are essential for prognosis and evaluation of early response to anti-fibrotic treatments.
Objectives: The aims of this study were to determine whether fluorodeoxyglucose ([18F]-FDG) lung uptake is reduced after initiation of pirfenidone or nintedanib and to assess its possible use as a prognostic factor.
Methods: [18F]-FDG PET/CT was performed in IPF patients and in a murine model of pulmonary fibrosis. PET/CTs were performed at day 8 and day 15 post-instillation of bleomycin in pirfenidone- or vehicule-treated mice. In IPF patients, PET-CT was performed before and 3 months after the initiation of pirfenidone or nintedanib.
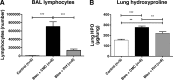
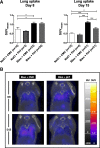
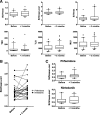
Results: In bleomycin-treated mice, pirfenidone significantly reduced the [18F]-FDG uptake compared to vehicule-treated mice at day 15 (p < 0.001), whereas no difference was observed at day 8 after bleomycin administration. In IPF patients, [18F]-FDG lung uptake before and after 3 months of treatment by nintedanib (n = 11) or pirfenidone (n = 14) showed no significant difference regardless the antifibrotic treatment. Moreover, no difference was noticed between patients with progressive or non-progressive disease at one year of follow up.
Conclusions: Pirfenidone significantly reduces the lung [18F]-FDG uptake during the fibrotic phase in a mouse model of IPF. However, these preclinical data were not confirmed in IPF patients 3 months after the initiation of antifibrotic therapy. [18F]-FDG seems therefore not useful in clinical practice to assess the early response of IPF patients to nintedanib or pirfenidone.
Keywords: Biomarker; ILD; IPF; Idiopathic pulmonary fibrosis; Interstitial lung disease; Nintedanib; PET/CT; Pirfenidone.
Conflict of interest statement
Ethics approval and consent to participate
Experiments in mice were approved by the local committee for animal welfare (CMMI).
For the human study, the protocol has been approved by the Erasme hospital Ethics Committee (ref. P2016/427). Written informed consent for participation in the study was obtained from all the patients.
Consent for publication
Not applicable.
Competing interests
B.B. received grants, consultancies and lecture fees from La Roche-Hoffmann and Boehringer Ingelheim. Other authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

Similar articles
-
Imaging Pulmonary Fibrosis and Treatment Efficacy In Vivo with Autotaxin-Specific PET Ligand [18F]ATX-1905.Mol Pharm. 2024 Oct 7;21(10):5171-5181. doi: 10.1021/acs.molpharmaceut.4c00571. Epub 2024 Aug 26. Mol Pharm. 2024. PMID: 39186477
-
[18F]FMISO PET/CT imaging of hypoxia as a non-invasive biomarker of disease progression and therapy efficacy in a preclinical model of pulmonary fibrosis: comparison with the [18F]FDG PET/CT approach.Eur J Nucl Med Mol Imaging. 2021 Sep;48(10):3058-3074. doi: 10.1007/s00259-021-05209-2. Epub 2021 Feb 13. Eur J Nucl Med Mol Imaging. 2021. PMID: 33580818 Free PMC article.
-
Differential effects of Nintedanib and Pirfenidone on lung alveolar epithelial cell function in ex vivo murine and human lung tissue cultures of pulmonary fibrosis.Respir Res. 2018 Sep 15;19(1):175. doi: 10.1186/s12931-018-0876-y. Respir Res. 2018. PMID: 30219058 Free PMC article.
-
Efficacy of antifibrotic drugs, nintedanib and pirfenidone, in treatment of progressive pulmonary fibrosis in both idiopathic pulmonary fibrosis (IPF) and non-IPF: a systematic review and meta-analysis.BMC Pulm Med. 2021 Dec 11;21(1):411. doi: 10.1186/s12890-021-01783-1. BMC Pulm Med. 2021. PMID: 34895203 Free PMC article.
-
Antifibrotic therapy for fibrotic lung disease beyond idiopathic pulmonary fibrosis.Eur Respir Rev. 2019 Oct 1;28(153):190022. doi: 10.1183/16000617.0022-2019. Print 2019 Sep 30. Eur Respir Rev. 2019. PMID: 31578210 Free PMC article. Review.
Cited by
-
The fibroblast activation protein alpha as a biomarker of pulmonary fibrosis.Front Med (Lausanne). 2024 Sep 19;11:1393778. doi: 10.3389/fmed.2024.1393778. eCollection 2024. Front Med (Lausanne). 2024. PMID: 39364020 Free PMC article. Review.
-
Imaging Biomarkers and Pathobiological Profiling in a Rat Model of Drug-Induced Interstitial Lung Disease Induced by Bleomycin.Front Physiol. 2020 Jun 19;11:584. doi: 10.3389/fphys.2020.00584. eCollection 2020. Front Physiol. 2020. PMID: 32636756 Free PMC article.
-
Progress and Research Priorities in Imaging Genomics for Heart and Lung Disease: Summary of an NHLBI Workshop.Circ Cardiovasc Imaging. 2021 Aug;14(8):e012943. doi: 10.1161/CIRCIMAGING.121.012943. Epub 2021 Aug 13. Circ Cardiovasc Imaging. 2021. PMID: 34387095 Free PMC article.
-
The lungs were on fire: a pilot study of 18F-FDG PET/CT in idiopathic-inflammatory-myopathy-related interstitial lung disease.Arthritis Res Ther. 2021 Jul 23;23(1):198. doi: 10.1186/s13075-021-02578-9. Arthritis Res Ther. 2021. PMID: 34301306 Free PMC article.
-
18F-AzaFol for Detection of Folate Receptor-β Positive Macrophages in Experimental Interstitial Lung Disease-A Proof-of-Concept Study.Front Immunol. 2019 Nov 22;10:2724. doi: 10.3389/fimmu.2019.02724. eCollection 2019. Front Immunol. 2019. PMID: 31824505 Free PMC article.
References
-
- Groves AM, Win T, Screaton NJ, Berovic M, Endozo R, Booth H, et al. Idiopathic pulmonary fibrosis and diffuse parenchymal lung disease: implications from initial experience with 18F-FDG PET/CT. J Nucl Med Off Publ Soc Nucl Med. 2009;50(4):538–545. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

