Prevalence and risk of progression of preclinical Alzheimer's disease stages: a systematic review and meta-analysis
- PMID: 30646955
- PMCID: PMC6334406
- DOI: 10.1186/s13195-018-0459-7
Prevalence and risk of progression of preclinical Alzheimer's disease stages: a systematic review and meta-analysis
Abstract
Background: Alzheimer's disease (AD) pathology begins several years before the clinical onset. The long preclinical phase is composed of three stages according to the 2011National Institute on Aging and Alzheimer's Association (NIA-AA) criteria, followed by mild cognitive impairment (MCI), a featured clinical entity defined as "due to AD", or "prodromal AD", when pathophysiological biomarkers (i.e., cerebrospinal fluid or positron emission tomography with amyloid tracer) are positive. In the clinical setting, there is a clear need to detect the earliest symptoms not yet fulfilling MCI criteria, in order to proceed to biomarker assessment for diagnostic definition, thus offering treatment with disease-modifying drugs to patients as early as possible. According to the available evidence, we thus estimated the prevalence and risk of progression at each preclinical AD stage, with special interest in Stage 3.
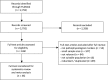
Methods: Cross-sectional and longitudinal studies published from April 2008 to May 2018 were obtained through MEDLINE-PubMed, screened, and systematically reviewed by four independent reviewers. Data from included studies were meta-analyzed using random-effects models. Heterogeneity was assessed by I2 statistics.
Results: Estimated overall prevalence of preclinical AD was 22% (95% CI = 18-26%). Rate of biomarker positivity overlapped in cognitively normal individuals and people with subjective cognitive decline. The risk of progression increases across preclinical AD stages, with individuals classified as NIA-AA Stage 3 showing the highest risk (73%, 95% CI = 40-92%) compared to those in Stage 2 (38%, 95% CI = 21-59%) and Stage 1 (20%, 95% CI = 10-34%).
Conclusion: Available data consistently show that risk of progression increases across the preclinical AD stages, where Stage 3 shows a risk of progression comparable to MCI due to AD. Accordingly, an effort should be made to also operationalize the diagnostic work-up in subjects with subtle cognitive deficits not yet fulfilling MCI criteria. The possibility to define, in the clinical routine, a patient as "pre-MCI due to AD" could offer these subjects the opportunity to use disease-modifying drugs at best.
Keywords: Biomarkers; National Institute on Aging–Alzheimer’s Association criteria; Preclinical Alzheimer’s disease; Prevalence; Systematic review.
Conflict of interest statement
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures



References
-
- Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, et al. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):280-92. - PMC - PubMed
-
- Dubois B, Feldman HH, Jacova C, Cummings JL, DeKosky ST, Barberger-Gateau P, et al. Revising the definition of Alzheimer’s disease: a new lexicon. Lancet Neurol. 2010;9(11):1118–1127. - PubMed
-
- Dubois B, Feldman HH, Jacova C, Hampel H, Molinuevo JL, Blennow K, et al. Advancing research diagnostic criteria for Alzheimer’s disease: the IWG-2 criteria. Lancet Neurol. 2014;13(6):614–629. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

