Tumor microenvironment modulation enhances immunologic benefit of chemoradiotherapy
- PMID: 30646957
- PMCID: PMC6332704
- DOI: 10.1186/s40425-018-0485-9
Tumor microenvironment modulation enhances immunologic benefit of chemoradiotherapy
Erratum in
-
Correction: Tumor microenvironment modulation enhances immunologic benefit of chemoradiotherapy.J Immunother Cancer. 2020 Mar;8(1):e0485-9corr. doi: 10.1136/jitc-2020-0485-9corr. J Immunother Cancer. 2020. PMID: 32152219 Free PMC article. No abstract available.
Abstract
Background: Chemoradiotherapy (CRT) remains one of the most common cancer treatment modalities, and recent data suggest that CRT is maximally effective when there is generation of an anti-tumoral immune response. However, CRT has also been shown to promote immunosuppressive mechanisms which must be blocked or reversed to maximize its immune stimulating effects.
Methods: Therefore, using a preclinical model of human papillomavirus (HPV)-associated head and neck squamous cell carcinoma (HNSCC), we developed a clinically relevant therapy combining CRT and two existing immunomodulatory drugs: cyclophosphamide (CTX) and the small molecule inducible nitric oxide synthase (iNOS) inhibitor L-n6-(1-iminoethyl)-lysine (L-NIL). In this model, we treated the syngeneic HPV-HNSCC mEER tumor-bearing mice with fractionated (10 fractions of 3 Gy) tumor-directed radiation and weekly cisplatin administration. We compared the immune responses induced by CRT and those induced by combinatory treatment (CRT + CTX/L-NIL) with flow cytometry, quantitative multiplex immunofluorescence and by profiling immune-related gene expression changes.
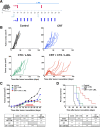
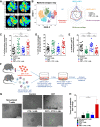
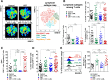
Results: We show that combination treatment favorably remodels the tumor myeloid immune microenvironment including an increase in anti-tumor immune cell types (inflammatory monocytes and M1-like macrophages) and a decrease in immunosuppressive granulocytic myeloid-derived suppressor cells (MDSCs). Intratumoral T cell infiltration and tumor antigen specificity of T cells were also improved, including a 31.8-fold increase in the CD8+ T cell/ regulatory T cell ratio and a significant increase in tumor antigen-specific CD8+ T cells compared to CRT alone. CTX/LNIL immunomodulation was also shown to significantly improve CRT efficacy, leading to rejection of 21% established tumors in a CD8-dependent manner.
Conclusions: Overall, these data show that modulation of the tumor immune microenvironment with CTX/L-NIL enhances susceptibility of treatment-refractory tumors to CRT. The combination of tumor immune microenvironment modulation with CRT constitutes a translationally relevant approach to enhance CRT efficacy through enhanced immune activation.
Keywords: Chemoradiotherapy; Cyclophosphamide; Head and neck cancer; Head and neck squamous cell carcinoma; Human papillomavirus (HPV); Immunotherapy; Inducible nitric oxide synthase (iNOS); L-n6-(1-iminoethyl)-lysine (L-NIL); Radiotherapy; Tumor microenvironment.
Conflict of interest statement
Ethics approval and consent to participate
All mice were housed and treated in accordance with Institutional Animal Care and Use Committee guidelines at Baylor College of Medicine. Patients were involved after signing informed consent and studies were conducted in accordance with the Declaration of Helsinki and approved by the local medical ethical committee of the Leiden University Medical Center (LUMC) and in agreement with the Dutch law.
Consent for publication
Not applicable.
Competing interests
R.D.G. has received travel support from PerkinElmer. A.G.S. receives support in the form of investigational drug from Advaxis for an investigator-initiated clinical trial.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures




References
-
- Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127(12):2893–2917. - PubMed
-
- Ndiaye C, Mena M, Alemany L, Arbyn M, Castellsagué X, Laporte L, Bosch FX, de Sanjosé S, Trottier H. HPV DNA, E6/E7 mRNA, and p16INK4a detection in head and neck cancers: a systematic review and meta-analysis. Lancet Oncol. 2014;15(12):1319–1331. - PubMed
-
- Bontkes HJ, de Gruijl TD, van den Muysenberg AJC, Verheijen RHM, Stukart MJ, Meijer CJLM, Scheper RJ, Stacey SN, Duggan-Keen MF, Stern PL, Man S, Borysiewicz LK, Walboomers JMM. Human papillomavirus type 16 E6/E7-specific cytotoxic T lymphocytes in women with cervical neoplasia. Int J Cancer. 2000;88(1):92–98. - PubMed
-
- Lassen P, Eriksen JG, Hamilton-Dutoit S, Tramm T, Alsner J, Overgaard J. Effect of HPV-associated p16INK4A expression on response to radiotherapy and survival in squamous cell carcinoma of the head and neck. J Clin Oncol. 2009;27(12):1992–1998. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
