Suppression of chemotherapy-induced cytokine/lipid mediator surge and ovarian cancer by a dual COX-2/sEH inhibitor
- PMID: 30647111
- PMCID: PMC6358686
- DOI: 10.1073/pnas.1803999116
Suppression of chemotherapy-induced cytokine/lipid mediator surge and ovarian cancer by a dual COX-2/sEH inhibitor
Abstract
Although chemotherapy is a conventional cancer treatment, it may induce a protumorigenic microenvironment by triggering the release of proinflammatory mediators. In this study, we demonstrate that ovarian tumor cell debris generated by first-line platinum- and taxane-based chemotherapy accelerates tumor progression by stimulating a macrophage-derived "surge" of proinflammatory cytokines and bioactive lipids. Thus, targeting a single inflammatory mediator or pathway is unlikely to prevent therapy-induced tumor progression. Here, we show that combined pharmacological abrogation of the cyclooxygenase-2 (COX-2) and soluble epoxide hydrolase (sEH) pathways prevented the debris-induced surge of both cytokines and lipid mediators by macrophages. In animal models, the dual COX-2/sEH inhibitor PTUPB delayed the onset of debris-stimulated ovarian tumor growth and ascites leading to sustained survival over 120 days postinjection. Therefore, dual inhibition of COX-2/sEH may be an approach to suppress debris-stimulated ovarian tumor growth by preventing the therapy-induced surge of cytokines and lipid mediators.
Keywords: cyclooxygenase; debris; inflammation; oxylipins; soluble epoxide hydrolase.
Conflict of interest statement
Conflict of interest statement: M.W.K. is now an employee of Bristol-Myers Squibb. His position at Bristol-Myers Squibb is not related to this work.
Figures

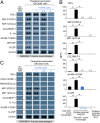
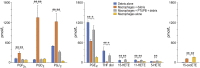


References
-
- Thibault B, Castells M, Delord JP, Couderc B. Ovarian cancer microenvironment: Implications for cancer dissemination and chemoresistance acquisition. Cancer Metastasis Rev. 2014;33:17–39. - PubMed
-
- Revesz L. Effect of tumour cells killed by X-rays upon the growth of admixed viable cells. Nature. 1956;178:1391–1392. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

