Human cytomegalovirus G protein-coupled receptor US28 promotes latency by attenuating c-fos
- PMID: 30647114
- PMCID: PMC6358704
- DOI: 10.1073/pnas.1816933116
Human cytomegalovirus G protein-coupled receptor US28 promotes latency by attenuating c-fos
Abstract
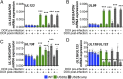
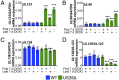
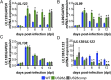
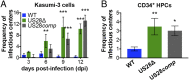
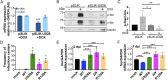
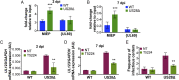
Human cytomegalovirus (HCMV) is a ubiquitous pathogen that undergoes latency in cells of the hematopoietic compartment, although the mechanisms underlying establishment and maintenance of latency remain elusive. We previously reported that the HCMV-encoded G protein-coupled receptor (GPCR) homolog US28 is required for successful latent infection. We now show that US28 protein (pUS28) provided in trans complements the US28Δ lytic phenotype in myeloid cells, suggesting that sustained US28 expression is necessary for long-term latency. Furthermore, expression of pUS28 at the time of infection represses transcription from the major immediate early promoter (MIEP) within 24 h. However, this repression is only maintained in the presence of continual pUS28 expression provided in trans Our data also reveal that pUS28-mediated signaling attenuates both expression and phosphorylation of cellular fos (c-fos), an AP-1 transcription factor subunit, to repress MIEP-driven transcription. AP-1 binds to the MIEP and promotes lytic replication, and in line with this we find that US28Δ infection results in an increase in AP-1 binding to the MIEP, compared with WT latent infection. Pharmacological inhibition of c-fos represses the MIEP during US28Δ infection to levels similar to those we observe during WT latent infection. Together, our data reveal that US28 is required for both establishment and long-term maintenance of HCMV latency, which is modulated, at least in part, by repressing functional AP-1 binding to the MIEP.
Keywords: HCMV; US28; cytomegalovirus; herpesvirus; latency.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
US28: HCMV's Swiss Army Knife.Viruses. 2018 Aug 20;10(8):445. doi: 10.3390/v10080445. Viruses. 2018. PMID: 30127279 Free PMC article. Review.
-
Latency-Associated Expression of Human Cytomegalovirus US28 Attenuates Cell Signaling Pathways To Maintain Latent Infection.mBio. 2017 Dec 5;8(6):e01754-17. doi: 10.1128/mBio.01754-17. mBio. 2017. PMID: 29208743 Free PMC article.
-
Activator protein-1 transactivation of the major immediate early locus is a determinant of cytomegalovirus reactivation from latency.Proc Natl Acad Sci U S A. 2020 Aug 25;117(34):20860-20867. doi: 10.1073/pnas.2009420117. Epub 2020 Aug 11. Proc Natl Acad Sci U S A. 2020. PMID: 32788362 Free PMC article.
-
Human Cytomegalovirus US28 Ligand Binding Activity Is Required for Latency in CD34+ Hematopoietic Progenitor Cells and Humanized NSG Mice.mBio. 2019 Aug 20;10(4):e01889-19. doi: 10.1128/mBio.01889-19. mBio. 2019. PMID: 31431555 Free PMC article.
-
HCMV latency: what regulates the regulators?Med Microbiol Immunol. 2019 Aug;208(3-4):431-438. doi: 10.1007/s00430-019-00581-1. Epub 2019 Feb 14. Med Microbiol Immunol. 2019. PMID: 30761409 Free PMC article. Review.
Cited by
-
Human cytomegalovirus and neonatal infection.Curr Res Microb Sci. 2024 Jun 24;7:100257. doi: 10.1016/j.crmicr.2024.100257. eCollection 2024. Curr Res Microb Sci. 2024. PMID: 39070527 Free PMC article. Review.
-
Cytomegalovirus Latency and Reactivation: An Intricate Interplay With the Host Immune Response.Front Cell Infect Microbiol. 2020 Mar 31;10:130. doi: 10.3389/fcimb.2020.00130. eCollection 2020. Front Cell Infect Microbiol. 2020. PMID: 32296651 Free PMC article. Review.
-
Herpesviral Latency-Common Themes.Pathogens. 2020 Feb 15;9(2):125. doi: 10.3390/pathogens9020125. Pathogens. 2020. PMID: 32075270 Free PMC article. Review.
-
Editorial: Cytomegalovirus Pathogenesis and Host Interactions.Front Cell Infect Microbiol. 2021 Jul 7;11:711551. doi: 10.3389/fcimb.2021.711551. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 34307201 Free PMC article. No abstract available.
-
CMV-encoded GPCR pUL33 activates CREB and facilitates its recruitment to the MIE locus for efficient viral reactivation.J Cell Sci. 2021 Jan 25;134(5):jcs254268. doi: 10.1242/jcs.254268. J Cell Sci. 2021. PMID: 33199520 Free PMC article.
References
-
- Mocarski ES, Courcelle CT. Cytomegaloviruses and Their Replication. 4th Ed Lippincott Williams & Wilkins; Philadelphia: 2001.
-
- Pass RF. Cytomegaloviruses. 4th Ed Lippincott Williams & Wilkins; Philadelphia: 2001.
-
- Khanna R, Diamond DJ. Human cytomegalovirus vaccine: Time to look for alternative options. Trends Mol Med. 2006;12:26–33. - PubMed
-
- Arvin AM, Fast P, Myers M, Plotkin S, Rabinovich R. National Vaccine Advisory Committee Vaccine development to prevent cytomegalovirus disease: Report from the National Vaccine Advisory Committee. Clin Infect Dis. 2004;39:233–239. - PubMed
-
- Griffiths P, Baraniak I, Reeves M. The pathogenesis of human cytomegalovirus. J Pathol. 2015;235:288–297. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

