Fluorescence resonance energy transfer links membrane ferroportin, hephaestin but not ferroportin, amyloid precursor protein complex with iron efflux
- PMID: 30647129
- PMCID: PMC6422103
- DOI: 10.1074/jbc.RA118.005142
Fluorescence resonance energy transfer links membrane ferroportin, hephaestin but not ferroportin, amyloid precursor protein complex with iron efflux
Abstract
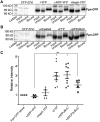
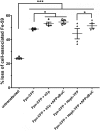
Iron efflux from mammalian cells is supported by the synergistic actions of the ferrous iron efflux transporter, ferroportin (Fpn) and a multicopper ferroxidase, that is, hephaestin (Heph), ceruloplasmin (Cp) or both. The two proteins stabilize Fpn in the plasma membrane and catalyze extracellular Fe3+ release. The membrane stabilization of Fpn is also stimulated by its interaction with a 22-amino acid synthetic peptide based on a short sequence in the extracellular E2 domain of the amyloid precursor protein (APP). However, whether APP family members interact with Fpn in vivo is unclear. Here, using cyan fluorescent protein (CFP)-tagged Fpn in conjunction with yellow fluorescent protein (YFP) fusions of Heph and APP family members APP, APLP1, and APLP2 in HEK293T cells we used fluorescence and surface biotinylation to quantify Fpn membrane occupancy and also measured 59Fe efflux. We demonstrate that Fpn and Heph co-localize, and FRET analysis indicated that the two proteins form an iron-efflux complex. In contrast, none of the full-length, cellular APP proteins exhibited Fpn co-localization or FRET. Moreover, iron supplementation increased surface expression of the iron-efflux complex, and copper depletion knocked down Heph activity and decreased Fpn membrane localization. Whereas cellular APP species had no effects on Fpn and Heph localization, addition of soluble E2 elements derived from APP and APLP2, but not APLP1, increased Fpn membrane occupancy. We conclude that a ferroportin-targeting sequence, (K/R)EWEE, present in APP and APLP2, but not APLP1, helps modulate Fpn-dependent iron efflux in the presence of an active multicopper ferroxidase.
Keywords: amyloid precursor protein (APP); ferroportin; fluorescence resonance energy transfer (FRET); hephaestin; iron efflux; iron metabolism; membrane transport; metal homeostasis.
© 2019 Dlouhy et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures








Comment in
-
APPealing for a role in cellular iron efflux.J Biol Chem. 2019 Jun 14;294(24):9365. doi: 10.1074/jbc.L119.009216. J Biol Chem. 2019. PMID: 31201240 Free PMC article. No abstract available.
Similar articles
-
The Ferroxidase Hephaestin But Not Amyloid Precursor Protein is Required for Ferroportin-Supported Iron Efflux in Primary Hippocampal Neurons.Cell Mol Neurobiol. 2018 May;38(4):941-954. doi: 10.1007/s10571-017-0568-z. Epub 2017 Nov 25. Cell Mol Neurobiol. 2018. PMID: 29177638 Free PMC article.
-
Ferroportin and exocytoplasmic ferroxidase activity are required for brain microvascular endothelial cell iron efflux.J Biol Chem. 2013 Jun 14;288(24):17932-40. doi: 10.1074/jbc.M113.455428. Epub 2013 May 2. J Biol Chem. 2013. PMID: 23640881 Free PMC article.
-
sAPP modulates iron efflux from brain microvascular endothelial cells by stabilizing the ferrous iron exporter ferroportin.EMBO Rep. 2014 Jul;15(7):809-15. doi: 10.15252/embr.201338064. Epub 2014 May 27. EMBO Rep. 2014. PMID: 24867889 Free PMC article.
-
Multi-copper oxidases and human iron metabolism.Nutrients. 2013 Jun 27;5(7):2289-313. doi: 10.3390/nu5072289. Nutrients. 2013. PMID: 23807651 Free PMC article. Review.
-
The cardinal roles of ferroportin and its partners in controlling cellular iron in and out.Life Sci. 2020 Oct 1;258:118135. doi: 10.1016/j.lfs.2020.118135. Epub 2020 Jul 24. Life Sci. 2020. PMID: 32712297 Review.
Cited by
-
Apo- and holo- transferrin differentially interact with ferroportin and hephaestin to regulate iron release at the blood-brain barrier.bioRxiv [Preprint]. 2023 Jan 10:2023.01.10.522344. doi: 10.1101/2023.01.10.522344. bioRxiv. 2023. Update in: J Biomed Sci. 2023 Jun 6;30(1):36. doi: 10.1186/s12929-023-00934-2. PMID: 36712094 Free PMC article. Updated. Preprint.
-
Brain Iron Dyshomeostasis and Ferroptosis in Alzheimer's Disease Pathophysiology: Two Faces of the Same Coin.Aging Dis. 2024 Jun 10;16(5):2615-2640. doi: 10.14336/AD.2024.0094. Aging Dis. 2024. PMID: 38913042 Free PMC article. Review.
-
Iron homeostasis imbalance and ferroptosis in brain diseases.MedComm (2020). 2023 Jun 26;4(4):e298. doi: 10.1002/mco2.298. eCollection 2023 Aug. MedComm (2020). 2023. PMID: 37377861 Free PMC article. Review.
-
Regional brain iron associated with deterioration in Alzheimer's disease: A large cohort study and theoretical significance.Alzheimers Dement. 2021 Jul;17(7):1244-1256. doi: 10.1002/alz.12282. Epub 2021 Jan 25. Alzheimers Dement. 2021. PMID: 33491917 Free PMC article.
-
Apo- and holo-transferrin differentially interact with hephaestin and ferroportin in a novel mechanism of cellular iron release regulation.J Biomed Sci. 2023 Jun 6;30(1):36. doi: 10.1186/s12929-023-00934-2. J Biomed Sci. 2023. PMID: 37277838 Free PMC article.
References
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

