The OMERACT-OARSI Core Domain Set for Measurement in Clinical Trials of Hip and/or Knee Osteoarthritis
- PMID: 30647185
- PMCID: PMC10753652
- DOI: 10.3899/jrheum.181194
The OMERACT-OARSI Core Domain Set for Measurement in Clinical Trials of Hip and/or Knee Osteoarthritis
Abstract
Objective: To update the 1997 OMERACT-OARSI (Outcome Measures in Rheumatology-Osteoarthritis Research Society International) core domain set for clinical trials in hip and/or knee osteoarthritis (OA).
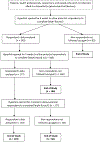
Methods: An initial review of the COMET database of core outcome sets (COS) was undertaken to identify all domains reported in previous COS including individuals with hip and/or knee OA. These were presented during 5 patient and health professionals/researcher meetings in 3 continents (Europe, Australasia, North America). A 3-round international Delphi survey was then undertaken among patients, healthcare professionals, researchers, and industry representatives to gain consensus on key domains to be included in a core domain set for hip and/or knee OA. Findings were presented and discussed in small groups at OMERACT 2018, where consensus was obtained in the final plenary.
Results: Four previous COS were identified. Using these, and the patient and health professionals/researcher meetings, 50 potential domains formed the Delphi survey. There were 426 individuals from 25 different countries who contributed to the Delphi exercise. OMERACT 2018 delegates (n = 129) voted on candidate domains. Six domains gained agreement as mandatory to be measured and reported in all hip and/or knee OA clinical trials: pain, physical function, quality of life, and patient's global assessment of the target joint, in addition to the mandated core domain of adverse events including mortality. Joint structure was agreed as mandatory in specific circumstances, i.e., depending on the intervention.
Conclusion: The updated core domain set for hip and/or knee OA has been agreed upon. Work will commence to determine which outcome measurement instrument should be recommended to cover each core domain.
Keywords: CLINICAL TRIALS; HIP JOINT; KNEE JOINT; OMERACT; OSTEOARTHRITIS; OUTCOME MEASURE.
Figures
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources