Bacterial persistence promotes the evolution of antibiotic resistance by increasing survival and mutation rates
- PMID: 30647458
- PMCID: PMC6474225
- DOI: 10.1038/s41396-019-0344-9
Bacterial persistence promotes the evolution of antibiotic resistance by increasing survival and mutation rates
Abstract
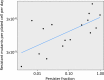
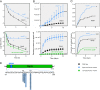
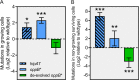
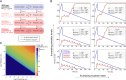
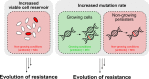
Persisters are transiently antibiotic-tolerant cells that complicate the treatment of bacterial infections. Both theory and experiments have suggested that persisters facilitate genetic resistance by constituting an evolutionary reservoir of viable cells. Here, we provide evidence for a strong positive correlation between persistence and the likelihood to become genetically resistant in natural and lab strains of E. coli. This correlation can be partly attributed to the increased availability of viable cells associated with persistence. However, our data additionally show that persistence is pleiotropically linked with mutation rates. Our theoretical model further demonstrates that increased survival and mutation rates jointly affect the likelihood of evolving clinical resistance. Overall, these results suggest that the battle against antibiotic resistance will benefit from incorporating anti-persister therapies.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
References
-
- World Health Organization. Antimicrobial resistance: global report on surveillance. WHO press, Geneva, Switzerland; 2014.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

