Involvement of M1 Macrophage Polarization in Endosomal Toll-Like Receptors Activated Psoriatic Inflammation
- PMID: 30647534
- PMCID: PMC6311781
- DOI: 10.1155/2018/3523642
Involvement of M1 Macrophage Polarization in Endosomal Toll-Like Receptors Activated Psoriatic Inflammation
Abstract
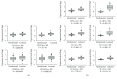
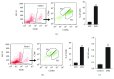
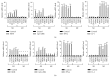
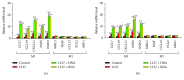
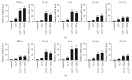
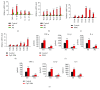
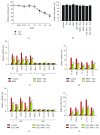
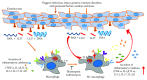
Psoriasis is a chronic inflammatory skin disorder that affects ~2%-3% of the worldwide population. Inappropriate and excessive activation of endosomal Toll-like receptors 7, 8, and 9 (TLRs 7-9) at the psoriatic site has been shown to play a pathogenic role in the onset of psoriasis. Macrophage is a major inflammatory cell type that can be differentiated into phenotypes M1 and M2. M1 macrophages produce proinflammatory cytokines, and M2 macrophages produce anti-inflammatory cytokines. The balance between these two types of macrophages determines the progression of various inflammatory diseases; however, whether macrophage polarization plays a role in psoriatic inflammation activated by endosomal TLRs has not been investigated. In this study, we investigated the function and mechanism of macrophages related to the pathogenic role of TLRs 7-9 in the progression of psoriasis. Analysis of clinical data in database revealed significantly increased expression of macrophage markers and inflammatory cytokines in psoriatic tissues over those in normal tissues. In animal studies, depletion of macrophages in mice ameliorated imiquimod, a TLR 7 agonist-induced psoriatic response. Imiquimod induced expression of genes and cytokines that are signature of M1 macrophage in the psoriatic lesions. In addition, treatment with this TLR 7 agonist shifted macrophages in the psoriatic lesions to a higher M1/M2 ratio. Both of the exogenous and endogenous TLR 7-9 ligands activated M1 macrophage polarization. M1 macrophages expressed higher levels of proinflammatory cytokines and TLRs 7-9 than M2 macrophages. These results suggest that by rendering macrophages into a more inflammatory status and capable of response to their ligands in the psoriatic sites, TLR 7-9 activation drives them to participate in endosomal TLR-activated psoriatic inflammation, resulting in an amplified inflammatory response. Our results also suggest that blocking M1 macrophage polarization could be a strategy which enables inhibition of psoriatic inflammation activated by these TLRs.
Figures

Similar articles
-
TLR2 stimulation impairs anti-inflammatory activity of M2-like macrophages, generating a chimeric M1/M2 phenotype.Arthritis Res Ther. 2017 Nov 2;19(1):245. doi: 10.1186/s13075-017-1447-1. Arthritis Res Ther. 2017. PMID: 29096690 Free PMC article.
-
Natural Modulators of Endosomal Toll-Like Receptor-Mediated Psoriatic Skin Inflammation.J Immunol Res. 2017;2017:7807313. doi: 10.1155/2017/7807313. Epub 2017 Aug 13. J Immunol Res. 2017. PMID: 28894754 Free PMC article. Review.
-
Conditional deletion of caspase-8 in macrophages alters macrophage activation in a RIPK-dependent manner.Arthritis Res Ther. 2015 Oct 16;17:291. doi: 10.1186/s13075-015-0794-z. Arthritis Res Ther. 2015. PMID: 26471282 Free PMC article.
-
Cervical cancer cell supernatants induce a phenotypic switch from U937-derived macrophage-activated M1 state into M2-like suppressor phenotype with change in Toll-like receptor profile.Biomed Res Int. 2014;2014:683068. doi: 10.1155/2014/683068. Epub 2014 Sep 21. Biomed Res Int. 2014. PMID: 25309919 Free PMC article.
-
The Role of M1/M2 Macrophage Polarization in Rheumatoid Arthritis Synovitis.Front Immunol. 2022 May 19;13:867260. doi: 10.3389/fimmu.2022.867260. eCollection 2022. Front Immunol. 2022. PMID: 35663975 Free PMC article. Review.
Cited by
-
A Guttate Psoriasis That Tends to Spare Three Tattoos: A Macrophage Liaison.Case Rep Dermatol Med. 2021 Sep 11;2021:9448636. doi: 10.1155/2021/9448636. eCollection 2021. Case Rep Dermatol Med. 2021. PMID: 34552797 Free PMC article.
-
Green Phellodendri Chinensis Cortex-based carbon dots for ameliorating imiquimod-induced psoriasis-like inflammation in mice.J Nanobiotechnology. 2021 Apr 15;19(1):105. doi: 10.1186/s12951-021-00847-y. J Nanobiotechnology. 2021. PMID: 33858431 Free PMC article.
-
Macrophage Functions in Psoriasis: Lessons from Mouse Models.Int J Mol Sci. 2024 May 13;25(10):5306. doi: 10.3390/ijms25105306. Int J Mol Sci. 2024. PMID: 38791342 Free PMC article. Review.
-
Macrophages in inflammatory skin diseases and skin tumors.Front Immunol. 2024 Dec 5;15:1430825. doi: 10.3389/fimmu.2024.1430825. eCollection 2024. Front Immunol. 2024. PMID: 39703508 Free PMC article. Review.
-
Recycled melanoma-secreted melanosomes regulate tumor-associated macrophage diversification.EMBO J. 2024 Sep;43(17):3553-3586. doi: 10.1038/s44318-024-00103-7. Epub 2024 May 8. EMBO J. 2024. PMID: 38719996 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

