Purification and characterization of thiol dependent, oxidation-stable serine alkaline protease from thermophilic Bacillus sp
- PMID: 30647567
- PMCID: PMC6299798
- DOI: 10.1016/j.jgeb.2015.01.002
Purification and characterization of thiol dependent, oxidation-stable serine alkaline protease from thermophilic Bacillus sp
Abstract
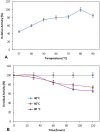
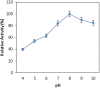
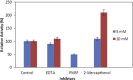
Alkaline serine protease was purified to homogeneity from culture supernatant of a thermophilic, alkaliphilic Bacillus sp. by 80% ammonium sulphate precipitation followed by CM-cellulose and DEAE-cellulose ion exchange column chromatography. The enzyme was purified up to 16.5-fold with 6900 U/mg activity. The protease exhibited maximum activity towards casein at pH 8.0 and at 80 °C. The enzyme was stable at pH 8.0 and 80 °C temperature up to 2 h. The Ca2+ and Mn2+ enhanced the proteolytic activity up to 44% and 36% as compared to control, respectively. However, Zn2+, K+, Ba2 +, Co2 +, Hg2+ and Cu2+ significantly reduced the enzyme activity. PMSF (phenyl methyl sulphonyl fluoride) completely inhibited the protease activity, whereas the activity of protease was stimulated up to two folds in the presence of 5 mM 2-mercaptoethanol. The enzyme was also stable in surfactant (Tween-80) and other commercial detergents (SDS, Triton X-100).
Keywords: Characterization; Oxidation-stable; Purification; Thermophilic serine protease; Thiol-dependent.
Figures

References
-
- M.S. Ammar, R.A. Bayoumi, A.M.H. El-Kasaby, A.M. Soliman, 5th Int Sci Conf, Al Azhar Univ, Fac Sci, Cairo, Egypt, 2003, pp. 54.
-
- Anwar A., Saleemuddin M. Bioresour. Technol. 1998;64:175–183.
-
- Banerjee U.C., Sani R.K., Azmi W., Soni R. Proc. Biochem. 1999;35:213–216.
-
- Beg Q.K., Sahai V., Gupta R. Proc. Biochem. 2003;39:203–206.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
