Inhibitory potential of important phytochemicals from Pergularia daemia (Forsk.) chiov., on snake venom (Naja naja)
- PMID: 30647617
- PMCID: PMC6299870
- DOI: 10.1016/j.jgeb.2015.11.002
Inhibitory potential of important phytochemicals from Pergularia daemia (Forsk.) chiov., on snake venom (Naja naja)
Abstract
Pergularia daemia (Forsk.) chiov., is a milk weed of Asclepiadaceae family. In the present study β-sitosterol, β-amyrin, α-amyrin and lupeol were identified in the leaf by GC-MS. Molecular docking studies were performed to evaluate their activities on phospholipase A2 (PLA2) and l-amino acid oxidase enzymes which constituted a rich source in snake venoms (Naja naja). Snake venom Phospholipase A2 with PDB code 1A3D devoid of co-crystallized ligand was extracted from Protein Data Bank. Using Molegro Virtual Docker two cavities are formed by cocrystallization. l-Amino acid oxidase (PDB code 4E0V) was a receptor model with a co-crystallized ligand FAD. Among the phytochemicals analysed, β-sitosterol displayed high affinity of binding to the active site regions of phospholipase A2 and l-amino acid oxidase, respectively. The affinity of binding was -125.939 and -157.521 kcal/mole identified by gold scores. α-Amyrin and β-amyrin had two hydrogen bond interactions with PLA2. Hence this study suggests that β-sitosterol identified in P. daemia can antagonize PLA2 and LAAO activities and forms a theoretical basis for the folk use of the plant against snake venom.
Keywords: Molecular docking; Pergularia daemia; Phospholipase A2; Snake venom; l-Amino acid oxidase.
Figures



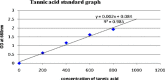
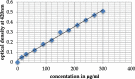


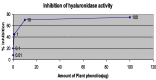

References
-
- Elango V., Ambujavalli L., Amala Basker E., Sulochana N. Fitoterapia. 1985;56:300–302.
-
- Singh V.P., Sharma S.K., Khare V.S. Indian Drugs Pharm. Ind. 1980;5:7–12.
-
- Dutta A., Ghosh S. J. Pharm. Sci. 1947;36:250–252. - PubMed
-
- Arseculeratne S.N., Gunatilaka A.A.L., Panabokke R.G. J. Ethnopharmacol. 1985;13(3) 323-3. - PubMed
-
- Selvanayahgam Z.E., Gnanevendhan S.G., Balakrishna K., Rao R.B. J. Herbs Spices Med. Plants. 1994;2:45–100.
LinkOut - more resources
Full Text Sources
Miscellaneous
