Follicle development as an orchestrated signaling network in a 3D organoid
- PMID: 30647770
- PMCID: PMC6327556
- DOI: 10.1186/s13036-018-0134-3
Follicle development as an orchestrated signaling network in a 3D organoid
Abstract
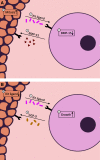
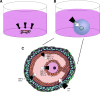
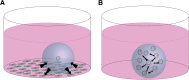
The ovarian follicle is the structural and functional unit of the ovary, composed of the female gamete (the oocyte) and supportive somatic cells. Follicles are not only the source of a female's germ cell supply, but also secrete important hormones necessary for proper endocrine function. Folliculogenesis, the growth and maturation of the follicular unit, is a complex process governed by both intrafollicular crosstalk and pituitary-secreted hormones. While the later stages of this process are gonadotropin-dependent, early folliculogenesis appears to be controlled by the ovarian microenvironment and intrafollicular paracrine and autocrine signaling. In vitro follicle culture remains challenging because of the limited knowledge of growth factors and other cytokines influencing early follicle growth. Here we discuss the current state of knowledge on paracrine and autocrine signaling influencing primary follicles as they develop into the antral stage. Given the importance of intrafollicular signaling and the ovarian microenvironment, we reviewed the current engineering approaches for in vitro follicle culture, including 3D systems using natural hydrogels such as alginate and synthetic hydrogels such as poly(ethylene glycol). Our discussion is focused on what drives the proliferation of granulosa cells, development of the thecal layer, and antrum formation-three processes integral to follicle growth up to the antral stage. Further research in this area may reveal the mechanisms behind these complex signaling relationships within the follicle, leading to more successful and physiologically-relevant in vitro culture methods that will translate well to clinical applications.
Keywords: 3D culture; Biomaterials; Crosstalk; Cytokines; Ovarian folliculogenesis.
Conflict of interest statement
Not applicable.Not applicable.The authors declare that they have no competing interests.Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

