Microenvironment-derived ADAM28 prevents cancer dissemination
- PMID: 30647853
- PMCID: PMC6324684
- DOI: 10.18632/oncotarget.26449
Microenvironment-derived ADAM28 prevents cancer dissemination
Abstract
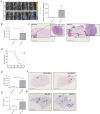
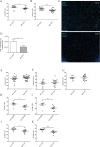
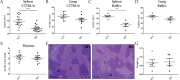
Previous studies have linked cancer cell-associated ADAM28 expression with tumor progression and metastatic dissemination. However, the role of host-derived ADAM28 in cancer dissemination processes remains unclear. Genetically engineered-mice fully deficient for ADAM28 unexpectedly display increased lung colonization by pulmonary, melanoma or breast tumor cells. In experimental tumor cell dissemination models, host ADAM28 deficiency is further associated with a decreased lung infiltration by CD8+ T lymphocytes. Notably, naive ADAM28-deficient mice already display a drastic reduction of CD8+ T cells in spleen which is further observed in lungs. Interestingly, ex vivo CD8+ T cell characterization revealed that ADAM28-deficiency does not impact proliferation, migration nor activation of CD8+ T cells. Our data highlight a functional role of ADAM28 in T cell mobilization and point to an unexpected protective role for host ADAM28 against metastasis.
Keywords: ADAM28; CD8+; T lymphocytes; lung; metastasis.
Conflict of interest statement
CONFLICTS OF INTEREST DC is the founder of Aquilon Pharmaceuticals, received speaker fees from AstraZeneca, Boehringer-Ingelheim, Novartis, MundiPharma, Chiesi and GSK and received consultancy fees from AstraZeneca, Boehringer-Ingelheim, and Novartis for the participation to advisory boards. None of these activities have any connection with oncology or development of drugs in the field of oncology.
Figures


References
-
- Mitsui Y, Mochizuki S, Kodama T, Shimoda M, Ohtsuka T, Shiomi T, Chijiiwa M, Ikeda T, Kitajima M, Okada Y. ADAM28 is overexpressed in human breast carcinomas: implications for carcinoma cell proliferation through cleavage of insulin-like growth factor binding protein-3. Cancer Res. 2006;66:9913–20. doi: 10.1158/0008-5472.CAN-06-0377. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

