Ir(iii)-catalyzed ortho C-H alkylations of (hetero)aromatic aldehydes using alkyl boron reagents
- PMID: 30647887
- PMCID: PMC6301268
- DOI: 10.1039/c8sc03606c
Ir(iii)-catalyzed ortho C-H alkylations of (hetero)aromatic aldehydes using alkyl boron reagents
Abstract
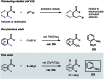
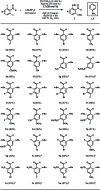
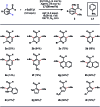
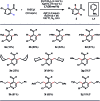
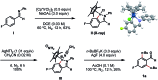
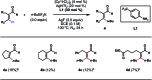
Transition-metal-catalyzed C-H alkylation reactions directed by aldehydes or ketones have been largely restricted to electronically activated alkenes. Herein, we report a general protocol for the Ir(iii)-catalyzed ortho C-H alkylations of (hetero)aromatic aldehydes using alkyl boron reagents as the coupling partner. Featuring aniline as an inexpensive catalytic ligand, the method was compatible with a wide variety of benzaldehydes, heterocyclic aldehydes, potassium alkyltrifluoroborates as well as a few α,β-unsaturated aldehydes. An X-ray crystal structure of a benzaldehyde ortho C-H iridation intermediate was also successfully obtained.
Figures
Similar articles
-
Ligand-accelerated ortho-C-H alkylation of arylcarboxylic acids using alkyl boron reagents.J Am Chem Soc. 2013 Nov 20;135(46):17508-13. doi: 10.1021/ja409014v. Epub 2013 Nov 8. J Am Chem Soc. 2013. PMID: 24124892 Free PMC article.
-
Ligand-enabled ruthenium-catalyzed meta-C-H alkylation of (hetero)aromatic carboxylic acids.Nat Commun. 2024 Jul 2;15(1):5552. doi: 10.1038/s41467-024-49362-2. Nat Commun. 2024. PMID: 38956019 Free PMC article.
-
Cross-coupling reaction of alkyl halides with grignard reagents catalyzed by Ni, Pd, or Cu complexes with pi-carbon ligand(s).Acc Chem Res. 2008 Nov 18;41(11):1545-54. doi: 10.1021/ar800138a. Acc Chem Res. 2008. PMID: 18973349
-
Experimental and Computational Studies on the Ruthenium-Catalyzed Dehydrative C-H Coupling of Phenols with Aldehydes for the Synthesis of 2-Alkylphenol, Benzofuran, and Xanthene Derivatives.J Am Chem Soc. 2021 Aug 25;143(33):13428-13440. doi: 10.1021/jacs.1c06887. Epub 2021 Aug 16. J Am Chem Soc. 2021. PMID: 34428913
-
Coinage Metal-Catalyzed Asymmetric Reactions of ortho-Alkynylaryl and Heteroaryl Aldehydes and Ketones.Molecules. 2022 Oct 17;27(20):6970. doi: 10.3390/molecules27206970. Molecules. 2022. PMID: 36296560 Free PMC article. Review.
Cited by
-
Lewis acid-assisted Ir(iii) reductive elimination enables construction of seven-membered-ring sulfoxides.Chem Sci. 2020 Sep 3;11(37):10149-10158. doi: 10.1039/d0sc04180g. Chem Sci. 2020. PMID: 34094278 Free PMC article.
-
Overcoming peri- and ortho-selectivity in C-H methylation of 1-naphthaldehydes by a tunable transient ligand strategy.Chem Sci. 2022 Jan 6;13(10):2900-2908. doi: 10.1039/d1sc05899a. eCollection 2022 Mar 9. Chem Sci. 2022. PMID: 35382469 Free PMC article.
-
Functionalization of C-H bonds in acetophenone oximes with arylacetic acids and elemental sulfur.RSC Adv. 2020 Mar 17;10(19):11024-11032. doi: 10.1039/d0ra00808g. eCollection 2020 Mar 16. RSC Adv. 2020. PMID: 35495317 Free PMC article.
-
The crucial role of silver(I)-salts as additives in C-H activation reactions: overall analysis of their versatility and applicability.Chem Soc Rev. 2023 Sep 18;52(18):6359-6378. doi: 10.1039/d3cs00328k. Chem Soc Rev. 2023. PMID: 37655711 Free PMC article. Review.
References
-
- Chen X., Engle K. M., Wang D.-H., Yu J.-Q. Angew. Chem., Int. Ed. 2009;48:5094–5115. - PMC - PubMed
- Ackermann L. Chem. Commun. 2010;46:4866–4877. - PubMed
- Colby D. A., Bergman R. G., Ellman J. A. Chem. Rev. 2010;110:624–655. - PMC - PubMed
- Lyons T. W., Sanford M. S. Chem. Rev. 2010;110:1147–1169. - PMC - PubMed
- Engle K. M., Mei T.-S., Wasa M., Yu J.-Q. Acc. Chem. Res. 2012;45:788–802. - PMC - PubMed
- He J., Wasa M., Chan K. S. L., Shao Q., Yu J.-Q. Chem. Rev. 2017;117:8754–8786. - PMC - PubMed
-
- Gürbüz N., Özdemir I., Çetinkaya B. Tetrahedron Lett. 2005;46:2273–2277.
- Padala K., Jeganmohan M. Org. Lett. 2012;14:1134–1137. - PubMed
- Lanke V., Ramaiah Prabhu K. Org. Lett. 2013;15:6262–6265. - PubMed
- Yang F. Z., Rauch K., Kettelhoit K., Ackermann L. Angew. Chem., Int. Ed. 2014;53:11285–11288. - PubMed
- Santhoshkumar R., Mannathan S., Cheng C.-H. J. Am. Chem. Soc. 2015;137:16116–16120. - PubMed
-
- Tremont S. J., Rahman H. U. J. Am. Chem. Soc. 1984;106:5759–5760.
- Chen X., Goodhue C. E., Yu J.-Q. J. Am. Chem. Soc. 2006;128:12634–12635. - PubMed
- Chen X., Li J.-J., Hao X.-S., Goodhue C. E., Yu J.-Q. J. Am. Chem. Soc. 2006;128:78–79. - PubMed
- Ackermann L., Novák P., Vicente R., Hofmann N. Angew. Chem., Int. Ed. 2009;48:6045–6048. - PubMed
- Zhang Y.-H., Shi B.-F., Yu J.-Q. Angew. Chem., Int. Ed. 2009;48:6097–6100. - PMC - PubMed
- Shabashov D., Daugulis O. J. Am. Chem. Soc. 2010;132:3965–3972. - PMC - PubMed
- Chen Q., Ilies L., Nakamura E. J. Am. Chem. Soc. 2011;133:428–429. - PubMed
- Zhao Y., Chen G. Org. Lett. 2011;13:4850–4853. - PubMed
- Aihara Y., Chatani N. J. Am. Chem. Soc. 2013;135:5308–5311. - PubMed
- Chen K., Hu F., Zhang S.-Q., Shi B.-F. Chem. Sci. 2013;4:3906–3911.
- Neufeldt S. R., Seigerman C. K., Sanford M. S. Org. Lett. 2013;15:2302–2305. - PMC - PubMed
- Thuy-Boun P. S., Villa G., Dang D., Richardson P., Su S., Yu J.-Q. J. Am. Chem. Soc. 2013;135:17508–17513. - PMC - PubMed
- Zhang S.-Y., He G., Nack W. A., Zhao Y., Li Q., Chen G. J. Am. Chem. Soc. 2013;135:2124–2127. - PubMed
- Zhang S.-Y., Li Q., He G., Nack W. A., Chen G. J. Am. Chem. Soc. 2013;135:12135–12141. - PubMed
- Chen K., Shi B.-F. Angew. Chem., Int. Ed. 2014;53:11950–11954. - PubMed
- Ilies L., Matsubara T., Ichikawa S., Asako S., Nakamura E. J. Am. Chem. Soc. 2014;136:13126–13129. - PubMed
- Zhu R.-Y., He J., Wang X.-C., Yu J.-Q. J. Am. Chem. Soc. 2014;136:13194–13197. - PMC - PubMed
- Wang H., Yu S., Qi Z., Li X. Org. Lett. 2015;17:2812–2815. - PubMed
- Wippich J., Schnapperelle I., Bach T. Chem. Commun. 2015;51:3166–3168. - PubMed
- Zhang S.-Y., Li Q., He G., Nack W. A., Chen G. J. Am. Chem. Soc. 2015;137:531–539. - PubMed
- Chen K., Li X., Zhang S.-Q., Shi B.-F. Chem. Commun. 2016;52:1915–1918. - PubMed
- Cera G., Haven T., Ackermann L. Angew. Chem., Int. Ed. 2016;55:1484–1488. - PubMed
- Peng P., Wang J., Jiang H., Liu H. Org. Lett. 2016;18:5376–5379. - PubMed
- Wiest J. M., Pothig A., Bach T. Org. Lett. 2016;18:852–855. - PubMed
- Chen Z., Hu L. a., Zeng F., Zhu R., Zheng S., Yu Q., Huang J. Chem. Commun. 2017;53:4258–4261. - PubMed
- Shao Q., He J., Wu Q.-F., Yu J.-Q. ACS Catal. 2017;7:7777–7782.
-
- Murai S., Kakiuchi F., Sekine S., Tanaka Y., Kamatani A., Sonoda M., Chatani N. Nature. 1993;366:529–531.
- Kakiuchi F., Yamauchi M., Chatani N., Murai S. Chem. Lett. 1996;25:111–112.
- Lenges C. P., Brookhart M. J. Am. Chem. Soc. 1999;121:6616–6623.
- Jun C.-H., Hong J.-B., Kim Y.-H., Chung K.-Y. Angew. Chem., Int. Ed. 2000;39:3440–3442. - PubMed
- Lim Y.-G., Han J.-S., Yang S.-S., Chun J. H. Tetrahedron Lett. 2001;42:4853–4856.
- Thalji R. K., Ahrendt K. A., Bergman R. G., Ellman J. A. J. Am. Chem. Soc. 2001;123:9692–9693. - PubMed
- Jun C.-H., Moon C. W., Hong J.-B., Lim S.-G., Chung K.-Y., Kim Y.-H. Chem.–Eur. J. 2002;8:485–492. - PubMed
- Thalji R. K., Ellman J. A., Bergman R. G. J. Am. Chem. Soc. 2004;126:7192–7193. - PubMed
- Martinez R., Chevalier R., Darses S., Genet J.-P. Angew. Chem., Int. Ed. 2006;45:8232–8235. - PubMed
- Watzke A., Wilson R. M., O'Malley S. J., Bergman R. G., Ellman J. A. Synlett. 2007;2007:2383–2389.
- Tsuchikama K., Kasagawa M., Hashimoto Y.-K., Endo K., Shibata T. J. Organomet. Chem. 2008;693:3939–3942.
- Kakiuchi F., Kochi T., Mizushima E., Murai S. J. Am. Chem. Soc. 2010;132:17741–17750. - PubMed
- Crisenza G. E. M., McCreanor N. G., Bower J. F. J. Am. Chem. Soc. 2014;136:10258–10261. - PubMed
- Kimura N., Kochi T., Kakiuchi F. J. Am. Chem. Soc. 2017;139:14849–14852. - PubMed
-
- Kakiuchi F., Tanaka Y., Sato T., Chatani N., Murai S. Chem. Lett. 1995;24:679–680.
- Trost B. M., Imi K., Davies I. W. J. Am. Chem. Soc. 1995;117:5371–5372.
- Sato T., Kakiuchi F., Chatani N., Murai S. Chem. Lett. 1998;27:893–894.
- Kakiuchi F., Sato T., Igi K., Chatani N., Murai S. Chem. Lett. 2001;30:386–387.
- Jun C.-H., Moon C. W., Kim Y.-M., Lee H., Lee J. H. Tetrahedron Lett. 2002;43:4233–4236.
- Colby D. A., Bergman R. G., Ellman J. A. J. Am. Chem. Soc. 2006;128:5604–5605. - PMC - PubMed
LinkOut - more resources
Full Text Sources

