Disulfiram repurposing combined with nutritional copper supplement as add-on to chemotherapy in recurrent glioblastoma (DIRECT): Study protocol for a randomized controlled trial
- PMID: 30647912
- PMCID: PMC6325620
- DOI: 10.12688/f1000research.16786.1
Disulfiram repurposing combined with nutritional copper supplement as add-on to chemotherapy in recurrent glioblastoma (DIRECT): Study protocol for a randomized controlled trial
Abstract
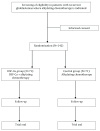
Background: Disulfiram (DSF) is a well-tolerated, inexpensive, generic drug that has been in use to treat alcoholism since the 1950s. There is now independent preclinical data that supports DSF as an anticancer agent, and experimental data suggest that copper may increase its anti-neoplastic properties. There is also some clinical evidence that DSF is a promising anticancer agent in extracranial cancers. In glioblastoma, DSF induced O 6-methylguanine methyltransferase (MGMT) inhibition may increase response to alkylating chemotherapy. A recent phase I study demonstrated the safety of DSF in glioblastoma patients when DSF was administered at doses below 500 mg/day together with chemotherapy. We plan to assess the effects of DSF combined with nutritional copper supplement (DSF-Cu) as an adjuvant to alkylating chemotherapy in glioblastoma treatment. Methods: In an academic, industry independent, multicenter, open label randomized controlled phase II/III trial with parallel group design (1:1) we will assess the efficacy and safety of DSF-Cu in glioblastoma treatment. The study will include 142 patients at the time of first recurrence of glioblastoma where salvage therapy with alkylating chemotherapy is planned. Patients will be randomized to treatment with or without DSF-Cu. Primary end-point is survival at 6 months. Secondary end-points are overall survival, progression free survival, quality of life, contrast enhancing tumor volume and safety. Discussion: There is a need to improve the treatment of recurrent glioblastoma. Results from this randomized controlled trial with DSF-Cu in glioblastoma will serve as preliminary evidence of the future role of DSF-Cu in glioblastoma treatment and a basis for design and power estimations of future studies. In this publication we provide rationale for our choices and discuss methodological issues. Trial registration: The study underwent registration in EudraCT 2016-000167-16 (Date: 30.03.2016,) and Clinicaltrials.gov NCT02678975 (Date: 31.01.2016) before initiating the study.
Keywords: Randomized controlled trial; alkylating agents; brain tumor; disulfiram; glioblastoma; glioma.
Conflict of interest statement
No competing interests were disclosed.
Figures
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials