Efficacy and Safety of the Combination Treatment of Rituximab and Dexamethasone for Adults with Primary Immune Thrombocytopenia (ITP): A Meta-Analysis
- PMID: 30648105
- PMCID: PMC6311778
- DOI: 10.1155/2018/1316096
Efficacy and Safety of the Combination Treatment of Rituximab and Dexamethasone for Adults with Primary Immune Thrombocytopenia (ITP): A Meta-Analysis
Abstract
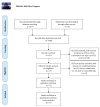
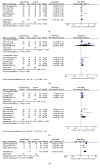
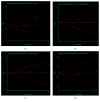
Objective. To conduct a meta-analysis, assessing the efficacy and safety of the combination treatment of dexamethasone and rituximab for adults with ITP (primary immune thrombocytopenia). Methods. Randomized controlled trials that compared rituximab and dexamethasone combination treatment to dexamethasone monotherapy in the treatment of adults with ITP were collected by searching Pubmed, Embase, Cochrane, China National Knowledge (CNKI), Wanfang database, and Sino Med. We conducted pooled analyses on OR (overall response) rate, CR (complete response) rate, PR (partial response) rate, SR (sustained response) rate, R (relapse) rate, change in Treg cell count (mean [SD]), and AE (adverse event). GRADE pro scale was used to assess the quality of the evidence. Publication bias was assessed with Egger's test method. Results. A total of 11 randomized controlled trials were eligible for inclusion. The overall efficacy estimates favored combination arm in terms of OR rate at month 3, CR rate at week 4 and month 3, SR rate, and Treg cell count at week 2. Subgroup analysis showed that females obtained a higher OR rate than males did at week 4. No significant difference was found in pooled analysis of relapse rate between combination arm and monotherapy arm. The comparison of serious AE and other AEs showed no significant difference either. A total of 19 outcomes were assessed by GRADE pro software, of which 79% (15/19) was scaled as moderate-to-high level. Publication bias existed in studies on OR at week 4 (P=0.025), CR at week 4 (P=0.017), infection (P=0.006), and rash (P=0.028) of the AEs. Conclusion. Dexamethasone combined with rituximab can provide a better long-term response in the treatment of adults with ITP and will not increase the risk of adverse effects.
Figures




Similar articles
-
Efficacy and safety of rituximab for minors with immune thrombocytopenia: a systematic review and meta-analysis.J Int Med Res. 2020 Oct;48(10):300060520962348. doi: 10.1177/0300060520962348. J Int Med Res. 2020. PMID: 33115308 Free PMC article.
-
Anti-GPIb/IX autoantibodies are associated with poor response to dexamethasone combined with rituximab therapy in primary immune thrombocytopenia patients.Platelets. 2023 Dec;34(1):2258988. doi: 10.1080/09537104.2023.2258988. Epub 2023 Sep 18. Platelets. 2023. PMID: 37722393
-
[Rituximab and Dexamethasone Combined with Cyclophosphamide for Treatment of Relapsed and Refractory Immune Thrombocytopenia].Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2016 Feb;24(1):162-6. doi: 10.7534/j.issn.1009-2137.2016.01.031. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2016. PMID: 26913414 Chinese.
-
Dexamethasone plus rituximab yields higher sustained response rates than dexamethasone monotherapy in adults with primary immune thrombocytopenia.Blood. 2010 Apr 8;115(14):2755-62. doi: 10.1182/blood-2009-07-229815. Epub 2010 Feb 3. Blood. 2010. PMID: 20130241 Clinical Trial.
-
The Efficacy of High-Dose Dexamethasone vs. Other Treatments for Newly Diagnosed Immune Thrombocytopenia: A Meta-Analysis.Front Med (Lausanne). 2021 May 25;8:656792. doi: 10.3389/fmed.2021.656792. eCollection 2021. Front Med (Lausanne). 2021. PMID: 34113634 Free PMC article.
Cited by
-
Short-term dose-escalated romiplostim for preparing an adult patient with persistent newly diagnosed primary immune thrombocytopenia for splenectomy.Hematol Transfus Cell Ther. 2020 Jul-Sep;42(3):283-286. doi: 10.1016/j.htct.2019.07.009. Epub 2019 Oct 10. Hematol Transfus Cell Ther. 2020. PMID: 31672588 Free PMC article. No abstract available.
-
Health-related quality of life and complications of corticosteroid treatment in patients with immune thrombocytopenia in two teaching hospitals in Ethiopia: a cross-sectional study.Front Med (Lausanne). 2024 Nov 5;11:1423161. doi: 10.3389/fmed.2024.1423161. eCollection 2024. Front Med (Lausanne). 2024. PMID: 39564500 Free PMC article.
-
Treatment outcomes and adherence to treatment in patients with immune thrombocytopenia in two Ethiopian teaching hospitals: a retrospective cohort study.Sci Rep. 2024 May 24;14(1):11917. doi: 10.1038/s41598-024-62372-w. Sci Rep. 2024. PMID: 38789461 Free PMC article.
-
Recent advances in treatments of adult immune thrombocytopenia.Blood Res. 2022 Apr 30;57(S1):112-119. doi: 10.5045/br.2022.2022038. Blood Res. 2022. PMID: 35483935 Free PMC article. Review.
-
Efficacy and safety of treatments in newly diagnosed adult primary immune thrombocytopenia: A systematic review and network meta-analysis.EClinicalMedicine. 2022 Dec 14;56:101777. doi: 10.1016/j.eclinm.2022.101777. eCollection 2023 Feb. EClinicalMedicine. 2022. PMID: 36578882 Free PMC article.
References
-
- Chong B. H., Lee J.-W., Jang J. H., et al. International ITP Registry with Focus on the Asia Pacific Region: Update on Preliminary Findings of Epidemiological and Clinical Data. Blood. 2017;130(1)
-
- Chong B. H., Lee J.-W., Jang J. H., et al. ITP Patients in the Asia Pacific: Are They Different? Blood. 2017;130(1)
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

