Critical Assessment of the Interaction between DNA and Choline Amino Acid Ionic Liquids: Evidences of Multimodal Binding and Stability Enhancement
- PMID: 30648148
- PMCID: PMC6311687
- DOI: 10.1021/acscentsci.8b00601
Critical Assessment of the Interaction between DNA and Choline Amino Acid Ionic Liquids: Evidences of Multimodal Binding and Stability Enhancement
Abstract
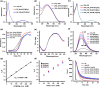
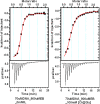
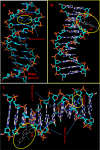
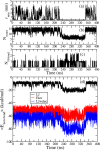
Long-term storage and stability of DNA is of paramount importance in biomedical applications. Ever since the emergence of ionic liquids (ILs) as alternate green solvents to aqueous and organic solvents, their exploration for the extraction and application of DNA need conscientious understanding of the binding characteristics and molecular interactions between IL and DNA. Choline amino acid ILs (CAAILs) in this regard seem to be promising due to their non-cytotoxic, completely biobased and environment-friendly nature. To unravel the key factors for the strength and binding mechanism of CAAILs with DNA, various spectroscopic techniques, molecular docking, and molecular dynamics simulations were employed in this work. UV-Vis spectra indicate multimodal binding of CAAILs with DNA, whereas dye displacement studies through fluorescence emission confirm the intrusion of IL molecules into the minor groove of DNA. Circular dichorism spectra show that DNA retains its native B-conformation in CAAILs. Both isothermal titration calorimetry and molecular docking studies provide an estimate of the binding affinity of DNA with CAAILs ≈ 4 kcal/mol. The heterogeneity in binding modes of CAAIL-DNA system with evolution of time was established by molecular dynamics simulations. Choline cation while approaching DNA first binds at surface through electrostatic interactions, whereas a stronger binding at minor groove occurs via van der Waals and hydrophobic interactions irrespective of anions considered in this study. We hope this result can encourage and guide the researchers in designing new bio-ILs for biomolecular studies in future.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

Similar articles
-
Critical assessment of interactions between ct-DNA and choline-based magnetic ionic liquids: evidences of compaction.Chem Sci. 2024 Mar 15;15(15):5507-5515. doi: 10.1039/d4sc00004h. eCollection 2024 Apr 17. Chem Sci. 2024. PMID: 38638223 Free PMC article.
-
Groove binding mechanism of ionic liquids: a key factor in long-term stability of DNA in hydrated ionic liquids?J Am Chem Soc. 2012 Dec 19;134(50):20330-9. doi: 10.1021/ja304519d. Epub 2012 Dec 10. J Am Chem Soc. 2012. PMID: 23181803
-
Effect of Choline Amino Acid-Based Ionic Liquids on Stability and Structure of Hemoglobin.Chemphyschem. 2023 Aug 1;24(15):e202300201. doi: 10.1002/cphc.202300201. Epub 2023 Jun 22. Chemphyschem. 2023. PMID: 37272734
-
Quantifying intermolecular interactions of ionic liquids using cohesive energy densities.R Soc Open Sci. 2017 Dec 6;4(12):171223. doi: 10.1098/rsos.171223. eCollection 2017 Dec. R Soc Open Sci. 2017. PMID: 29308254 Free PMC article. Review.
-
Ionic deep eutectic solvents for the extraction and separation of natural products.J Chromatogr A. 2019 Aug 2;1598:1-19. doi: 10.1016/j.chroma.2019.03.046. Epub 2019 Mar 30. J Chromatogr A. 2019. PMID: 31005289 Review.
Cited by
-
Hyper-Branched Cationic Cyclodextrin Polymers for Improving Plasmid Transfection in 2D and 3D Spheroid Cells.Pharmaceutics. 2022 Dec 1;14(12):2690. doi: 10.3390/pharmaceutics14122690. Pharmaceutics. 2022. PMID: 36559184 Free PMC article.
-
Gram-Scale Synthesis of 1,8-Naphthyridines in Water: The Friedlander Reaction Revisited.ACS Omega. 2021 Jul 12;6(29):19304-19313. doi: 10.1021/acsomega.1c02798. eCollection 2021 Jul 27. ACS Omega. 2021. PMID: 34337267 Free PMC article.
-
Anion-Driven Influence of Tetrabutylammonium-Based Ionic Liquids on DNA Stability and Interaction Mechanisms.ACS Phys Chem Au. 2025 Apr 22;5(4):387-397. doi: 10.1021/acsphyschemau.5c00015. eCollection 2025 Jul 23. ACS Phys Chem Au. 2025. PMID: 40727225 Free PMC article.
-
Pressure-Dependent Stability of Imidazolium-Based Ionic Liquid/DNA Materials Investigated by High-Pressure Infrared Spectroscopy.Materials (Basel). 2019 Dec 13;12(24):4202. doi: 10.3390/ma12244202. Materials (Basel). 2019. PMID: 31847290 Free PMC article.
-
Therapeutic mechanism of baicalein in peritoneal dialysis-associated peritoneal fibrosis based on network pharmacology and experimental validation.Front Pharmacol. 2023 May 17;14:1153503. doi: 10.3389/fphar.2023.1153503. eCollection 2023. Front Pharmacol. 2023. PMID: 37266145 Free PMC article.
References
LinkOut - more resources
Full Text Sources

