Myosin VIIa Supports Spermatid/Organelle Transport and Cell Adhesion During Spermatogenesis in the Rat Testis
- PMID: 30649248
- PMCID: PMC6372944
- DOI: 10.1210/en.2018-00855
Myosin VIIa Supports Spermatid/Organelle Transport and Cell Adhesion During Spermatogenesis in the Rat Testis
Abstract
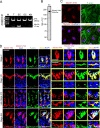
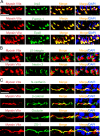
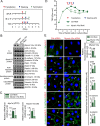
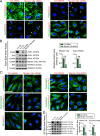
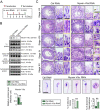
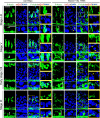
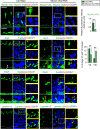
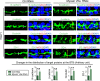
The biology of transport of spermatids and spermatid adhesion across the seminiferous epithelium during the epithelial cycle remains largely unexplored. Nonetheless, studies have implicated the role of motor proteins in these cellular events. In this article, we report findings to unravel the role of myosin VIIa, an F-actin-based barbed (+)-end-directed motor protein, to support cellular transport and adhesion in the testis. Using RNA interference to knock down myosin VIIa in Sertoli cells cultured in vitro as a study model was shown to perturb the Sertoli cell tight junction permeability barrier, mediated through disorganization of actin- or microtubule (MT)-based cytoskeletons owing to disruptive changes on the spatiotemporal expression of F-actin or MT-regulatory proteins. Consistent with these in vitro findings, knockdown of myosin VIIa in the testis in vivo also induced disorganization of the actin- and MT-based cytoskeletons across the seminiferous epithelium, mediated by disruptive changes in the spatiotemporal expression of actin- and MT-based regulatory proteins. More important, the transport of spermatids and organelles across the epithelium, as well as cell adhesion, was grossly disrupted. For instance, step 19 spermatids failed to be transported to the adluminal compartment near the tubule lumen to undergo spermiation; in this manner, step 19 spermatids were persistently detected in stage IX and XII tubules, intermingling with step 9 and 12 spermatids, respectively. Also, phagosomes were detected near the tubule lumen in stage I to III tubules when they should have been degraded near the base of the seminiferous epithelium via the lysosomal pathway. In summary, myosin VIIa motor protein was crucial to support cellular transport and adhesion during spermatogenesis.
Figures
Similar articles
-
KIF15 supports spermatogenesis via its effects on Sertoli cell microtubule, actin, vimentin, and septin cytoskeletons.Endocrinology. 2021 Apr 1;162(4):bqab010. doi: 10.1210/endocr/bqab010. Endocrinology. 2021. PMID: 33453102 Free PMC article.
-
Coordination of Actin- and Microtubule-Based Cytoskeletons Supports Transport of Spermatids and Residual Bodies/Phagosomes During Spermatogenesis in the Rat Testis.Endocrinology. 2016 Apr;157(4):1644-59. doi: 10.1210/en.2015-1962. Epub 2016 Feb 19. Endocrinology. 2016. PMID: 26894662 Free PMC article.
-
Ezrin is an actin binding protein that regulates sertoli cell and spermatid adhesion during spermatogenesis.Endocrinology. 2014 Oct;155(10):3981-95. doi: 10.1210/en.2014-1163. Epub 2014 Jul 22. Endocrinology. 2014. PMID: 25051438 Free PMC article.
-
Transport of germ cells across the seminiferous epithelium during spermatogenesis-the involvement of both actin- and microtubule-based cytoskeletons.Tissue Barriers. 2016 Nov 28;4(4):e1265042. doi: 10.1080/21688370.2016.1265042. eCollection 2016. Tissue Barriers. 2016. PMID: 28123928 Free PMC article. Review.
-
Regulation of microtubule (MT)-based cytoskeleton in the seminiferous epithelium during spermatogenesis.Semin Cell Dev Biol. 2016 Nov;59:35-45. doi: 10.1016/j.semcdb.2016.01.004. Epub 2016 Jan 11. Semin Cell Dev Biol. 2016. PMID: 26791048 Free PMC article. Review.
Cited by
-
NC1-Peptide From Collagen α3 (IV) Chains in the Basement Membrane of Testes Regulates Spermatogenesis via p-FAK-Y407.Endocrinology. 2020 Oct 1;161(10):bqaa133. doi: 10.1210/endocr/bqaa133. Endocrinology. 2020. PMID: 32761085 Free PMC article.
-
Loss of myosin VI expression affects acrosome/acroplaxome complex morphology during mouse spermiogenesis†.Biol Reprod. 2020 Aug 21;103(3):521-533. doi: 10.1093/biolre/ioaa071. Biol Reprod. 2020. PMID: 32412041 Free PMC article.
-
Myosin VI maintains the actin-dependent organization of the tubulobulbar complexes required for endocytosis during mouse spermiogenesis†‡.Biol Reprod. 2020 Apr 15;102(4):863-875. doi: 10.1093/biolre/ioz232. Biol Reprod. 2020. PMID: 31901088 Free PMC article.
-
KIF15 supports spermatogenesis via its effects on Sertoli cell microtubule, actin, vimentin, and septin cytoskeletons.Endocrinology. 2021 Apr 1;162(4):bqab010. doi: 10.1210/endocr/bqab010. Endocrinology. 2021. PMID: 33453102 Free PMC article.
-
Diverse functions of myosin VI in spermiogenesis.Histochem Cell Biol. 2021 Mar;155(3):323-340. doi: 10.1007/s00418-020-01954-x. Epub 2021 Jan 2. Histochem Cell Biol. 2021. PMID: 33386429 Free PMC article. Review.
References
-
- de Kretser DM, Kerr JB. The cytology of the testis In: Knobil E, Neill JB, Ewing LL, Greenwald GS, Markert CL, Pfaff DW, eds. The Physiology of Reproduction. Vol 1. New York, NY: Raven Press; 1988:837–932.
-
- O’Donnell L, Meachem SJ, Stanton PG, McLachlan RI. Endocrine regulation of spermatogenesis In: Neill JD, ed. Physiology of Reproduction. 3rd ed.Amsterdam: Elsevier; 2006:1017–1069.
-
- McLachlan RI, O’Donnell L, Meachem SJ, Stanton PG, de K, Pratis K, Robertson DM. Hormonal regulation of spermatogenesis in primates and man: insights for development of the male hormonal contraceptive. J Androl. 2002;23(2):149–162. - PubMed

