Evidence-Based Study Design for Hospital-Acquired Bacterial Pneumonia and Ventilator-Associated Bacterial Pneumonia
- PMID: 30649434
- PMCID: PMC6562159
- DOI: 10.1093/infdis/jiy578
Evidence-Based Study Design for Hospital-Acquired Bacterial Pneumonia and Ventilator-Associated Bacterial Pneumonia
Abstract
Background: The US Food and Drug Administration solicited evidence-based recommendations to improve guidance for studies of hospital-acquired bacterial pneumonia (HABP) and ventilator-associated bacterial pneumonia (VABP).
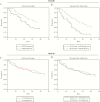
Methods: We analyzed 7 HABP/VABP datasets to explore novel noninferiority study endpoints and designs, focusing on alternatives to all-cause mortality (ACM).
Results: ACM at day 28 differed for ventilated HABP (27.8%), VABP (18.0%), and nonventilated HABP (14.5%). A "mortality-plus" (ACM+) composite endpoint was constructed by combining ACM with patient-relevant, infection-related adverse events from the Medical Dictionary for Regulatory Activities toxic/septic shock standardized query. The ACM+ rate was 3-10 percentage points above that of ACM across the studies and treatment groups. Predictors of higher ACM/ACM+ rates included older age and elevated acute physiology and chronic health evaluation (APACHE) II score. Only patients in the nonventilated HABP group were able to report pneumonia symptom changes.
Conclusions: If disease groups and patient characteristics in future studies produce an ACM rate so low (<10%-15%) that a fixed noninferiority margin of 10% cannot be justified (requiring an odds ratio analysis), an ACM+ endpoint could lower sample size. Enrichment of studies with patients with a higher severity of illness would increase ACM. Data on symptom resolution in nonventilated HABP support development of a patient-reported outcome instrument.
Keywords: all-cause mortality; hospital-acquired bacterial pneumonia; mortality-plus endpoint; ventilator-associated bacterial pneumonia.
© The Author(s) 2019. Published by Oxford University Press for the Infectious Diseases Society of America. All rights reserved. For permissions, e-mail: journals.permissions@oup.com.
Figures

Comment in
-
Exploring Standard Endpoints for Clinical Trials of Pneumonia Therapy.J Infect Dis. 2019 Apr 19;219(10):1515-1517. doi: 10.1093/infdis/jiy708. J Infect Dis. 2019. PMID: 30535391 No abstract available.
References
-
- Foundation for the National Institutes of Health Biomarkers Consortium HABP/VABP Working Group. Interim considerations for clinical trial design for the study of hospital-acquired bacterial pneumonia and ventilator-associated bacterial pneumonia. July 15, 2013, submission to docket #FDA-2013-N-0556 https://www.pharmamedtechbi.com/~/media/Supporting%20Documents/The%20Pin.... Accessed 2 April 2018.
-
- Biomarkers Consortium of the Foundation for the National Institutes of Health HABP-VABP Project Team. Considerations for clinical trial design for the study of hospital-acquired bacterial pneumonia and ventilator-associated bacteria pneumonia to the FDA docket for HABP/VABP draft guidance, June 13, 2017, submission to docket number FDA-2010-D-0589 https://www.regulations.gov/document?D=FDA-2010-D-0589-0027. Accessed 2 April 2018.
-
- Talbot GH, Powers JH, Hoffmann SC; Biomarkers Consortium of the Foundation for the National Institutes of Health CABP-ABSSSI and HABP-VABP Project Teams Developing outcomes assessments as endpoints for registrational clinical trials of antibacterial drugs: 2015 update from the biomarkers consortium of the foundation for the National Institutes of Health. Clin Infect Dis 2016; 62:603–7. - PMC - PubMed
-
- Kaul S, Diamond GA. Good enough: a primer on the analysis and interpretation of noninferiority trials. Ann Intern Med 2006; 145:62–9. - PubMed

