Effects of concomitant glucocorticoids in TOZURA, a common-framework study programme of subcutaneous tocilizumab in rheumatoid arthritis
- PMID: 30649524
- PMCID: PMC6532446
- DOI: 10.1093/rheumatology/key393
Effects of concomitant glucocorticoids in TOZURA, a common-framework study programme of subcutaneous tocilizumab in rheumatoid arthritis
Abstract
Objectives: This post hoc analysis of the TOZURA study programme evaluated the efficacy and safety of subcutaneous tocilizumab (TCZ-SC) as monotherapy or with concomitant conventional synthetic DMARDs (csDMARDs) in patients with RA categorized by baseline glucocorticoid (GC) use.
Methods: TOZURA was a multinational, open-label, single-arm, common-framework study programme (11 protocols, 22 countries) in patients with moderate to severe RA in whom csDMARDs or biologic therapies had failed or who were MTX naïve. Patients received once-weekly TCZ-SC 162 mg for ⩾24 weeks as monotherapy or in combination with csDMARDs and/or oral GC use (⩽10 mg/day prednisone or equivalent), which was to be continued unchanged for 24 weeks. Treatment subgroups were defined by baseline GC use and analysed for efficacy and safety.
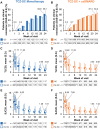
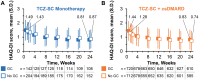
Results: Of 1804 patients who received TCZ-SC, 145 received monotherapy + GC, 208 received monotherapy without GC, 730 received combination therapy + GC and 721 received combination therapy without GC. The median GC dose in both GC subgroups was 5 mg/day. The proportion of patients who achieved clinical remission, defined as DAS in 28 joints using ESR <2.6, increased similarly from baseline to week 24 in all subgroups. Improvements in patient-reported outcomes were similar in all subgroups. Overall adverse event profiles were generally similar between subgroups, with some slight numerical differences between GC and non-GC subgroups.
Conclusion: The incremental efficacy benefits of TCZ-SC as monotherapy and in combination with csDMARDs were similar between patients with and without previous and continued oral GC treatment, with generally similar safety profiles.
Trial registration: ClinicalTrials.gov, http://www.clinicaltrials.gov, NCT01941940, NCT01941095, NCT01951170, NCT01987479, NCT01988012, NCT01995201, NCT02001987, NCT02011334, NCT02031471, NCT02046603, NCT02046616.
Keywords: RA; biologic therapies; csDMARDs; glucocorticoid; tocilizumab.
© The Author(s) 2019. Published by Oxford University Press on behalf of the British Society for Rheumatology.
Figures

References
-
- Scott DL, Wolfe F, Huizinga TW.. Rheumatoid arthritis. Lancet 2010;376:1094–108. - PubMed
-
- Strand V, Khanna D.. The impact of rheumatoid arthritis and treatment on patients’ lives. Clin Exp Rheumatol 2010;28:S32–40. - PubMed
-
- Caplan L, Wolfe F, Russell AS, Michaud K.. Corticosteroid use in rheumatoid arthritis: prevalence, predictors, correlates, and outcomes. J Rheumatol 2007;34:696–705. - PubMed
-
- Best JH, Kong AM, Lenhart GM. et al. Association between glucocorticoid exposure and healthcare expenditures for potential glucocorticoid-related adverse events in patients with rheumatoid arthritis. J Rheumatol 2018;45:320–8. - PubMed
-
- Wilson JC, Sarsour K, Gale S. et al. Incidence and risk of glucocorticoid-associated adverse effects in patients with rheumatoid arthritis. Arthritis Care Res (Hoboken) 2018; Advance Access published 1 June 2018, doi: 10.1002/acr.23611. - PubMed

