Dual binding motifs underpin the hierarchical association of perilipins1-3 with lipid droplets
- PMID: 30649995
- PMCID: PMC6589688
- DOI: 10.1091/mbc.E18-08-0534
Dual binding motifs underpin the hierarchical association of perilipins1-3 with lipid droplets
Abstract
Lipid droplets (LDs) in all eukaryotic cells are coated with at least one of the perilipin (Plin) family of proteins. They all regulate key intracellular lipases but do so to significantly different extents. Where more than one Plin is expressed in a cell, they associate with LDs in a hierarchical manner. In vivo, this means that lipid flux control in a particular cell or tissue type is heavily influenced by the specific Plins present on its LDs. Despite their early discovery, exactly how Plins target LDs and why they displace each other in a "hierarchical" manner remains unclear. They all share an amino-terminal 11-mer repeat (11mr) amphipathic region suggested to be involved in LD targeting. Here, we show that, in vivo, this domain functions as a primary highly reversible LD targeting motif in Plin1-3, and, in vitro, we document reversible and competitive binding between a wild-type purified Plin1 11mr peptide and a mutant with reduced binding affinity to both "naked" and phospholipid-coated oil-water interfaces. We also present data suggesting that a second carboxy-terminal 4-helix bundle domain stabilizes LD binding in Plin1 more effectively than in Plin2, whereas it weakens binding in Plin3. These findings suggest that dual amphipathic helical regions mediate LD targeting and underpin the hierarchical binding of Plin1-3 to LDs.
Figures


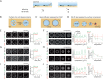
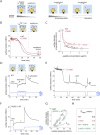
References
-
- Arimura N, Horiba T, Imagawa M, Shimizu M, Sato R. (2004). The peroxisome proliferator-activated receptor gamma regulates expression of the perilipin gene in adipocytes. J Biol Chem , 10070–10076. - PubMed
-
- Beller M, Bulankina AV, Hsiao HH, Urlaub H, Jackle H, Kuhnlein RP. (2010). PERILIPIN-dependent control of lipid droplet structure and fat storage in Drosophila. Cell Metab , 521–532. - PubMed
-
- Ben M’barek K, Ajjaji D, Chorlay A, Vanni S, Foret L, Thiam AR. (2017). ER membrane phospholipids and surface tension control cellular lipid droplet formation. Dev Cell , 591–604 e597. - PubMed
-
- Brasaemle DL. (2007). Thematic review series: adipocyte biology. The perilipin family of structural lipid droplet proteins: stabilization of lipid droplets and control of lipolysis. J Lipid Res , 2547–2559. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

