Acute HIV Infection in Youth: Protocol for the Adolescent Trials Network 147 (ATN147) Comprehensive Adolescent Research and Engagement Studies (CARES) Study
- PMID: 30650057
- PMCID: PMC6351983
- DOI: 10.2196/10807
Acute HIV Infection in Youth: Protocol for the Adolescent Trials Network 147 (ATN147) Comprehensive Adolescent Research and Engagement Studies (CARES) Study
Abstract
Background: Early treatment studies have shown that prompt treatment of HIV with combination antiretroviral therapy (cART) can limit the size of latent viral reservoirs, thereby providing clinical and public health benefits. Studies have demonstrated that adolescents have a greater capacity for immune reconstitution than adults. Nevertheless, adolescents who acquired HIV through sexual transmission have not been included in early treatment studies because of challenges in identification and adherence to cART.
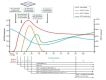
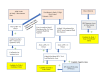
Objective: This study aimed to identify and promptly treat with cART youth aged 12 to 24 years in Los Angeles and New Orleans who have acute, recent, or established HIV infection, as determined by Fiebig stages 1 to 6 determined by viral RNA polymerase chain reaction, p24 antigen presence, and HIV-1 antigen Western blot. The protocol recommends treatment on the day of diagnosis when feasible. Surveillance and dedicated behavioral strategies are used to retain them in care and optimize adherence. Through serial follow-up, HIV biomarkers and response to antiretroviral therapy (ART) are assessed. The study aims to assess viral dynamics, decay and persistence of viral reservoirs over time, and correlate these data with the duration of viral suppression.
Methods: A total of 72 youth (36 acutely infected and 36 treatment naïve controls) are enrolled across clinical sites using a current community-based strategy and direct referrals. Youth are prescribed ART according to the standard of care HIV-1 management guidelines and followed for a period of 2 years. Assessments are conducted at specific time points throughout these 2 years of follow-up for monitoring of adherence to ART, viral load, magnitude of HIV reservoirs, and presence of coinfections.
Results: The study began enrolling youth in July 2017 across study sites in Los Angeles and New Orleans. As of September 30, 2018, a total of 37 youth were enrolled, 12 with recently acquired, 16 with established HIV infection as determined by Fiebig staging, and 9 pending determination of Fiebig status. Recruitment and enrollment are ongoing.
Conclusions: We hypothesize that the size of the HIV reservoir and immune activation markers will be different across groups treated with cART, that is, those with acute or recent HIV infection and those with established infection. Adolescents treated early who are virally suppressed will have diminished HIV reservoirs than those with established infection. These youth may be potential candidates for a possible HIV vaccine and additional HIV remission intervention trials. Our study will inform future studies of viral remission strategies.
International registered report identifier (irrid): DERR1-10.2196/10807.
Keywords: HIV; adolescents; youth.
©Karin Nielsen-Saines, Kate Mitchell, Tara Kerin, Jasmine Fournier, Leslie Kozina, Brenda Andrews, Ruth Cortado, Robert Bolan, Risa Flynn, Mary Jane Rotheram, Sue Ellen Abdalian, Yvonne Bryson, Adolescent Medicine Trials Network (ATN) CARES Team. Originally published in JMIR Research Protocols (http://www.researchprotocols.org), 16.01.2019.
Conflict of interest statement
Conflicts of Interest: None declared.
Figures
Similar articles
-
Demographics of Youth With Newly Diagnosed Acute/Recent HIV Infection in Adolescent Trials Network 147: Early Treatment of Acute HIV Infection.J Adolesc Health. 2024 Mar;74(3):573-581. doi: 10.1016/j.jadohealth.2023.09.017. Epub 2023 Dec 3. J Adolesc Health. 2024. PMID: 38043041 Free PMC article.
-
Recruitment of Youth Living With HIV to Optimize Adherence and Virologic Suppression: Testing the Design of Technology-Based Community Health Nursing to Improve Antiretroviral Therapy (ART) Clinical Trials.JMIR Res Protoc. 2020 Dec 11;9(12):e23480. doi: 10.2196/23480. JMIR Res Protoc. 2020. PMID: 33306036 Free PMC article.
-
The Stepped Care Intervention to Suppress Viral Load in Youth Living With HIV: Protocol for a Randomized Controlled Trial.JMIR Res Protoc. 2019 Feb 27;8(2):e10791. doi: 10.2196/10791. JMIR Res Protoc. 2019. PMID: 30810536 Free PMC article.
-
Molecular biological assessment methods and understanding the course of the HIV infection.APMIS Suppl. 2003;(114):1-37. APMIS Suppl. 2003. PMID: 14626050 Review.
-
Interventions to Improve Antiretroviral Therapy Adherence Among Adolescents and Youth in Low- and Middle-Income Countries: A Systematic Review 2015-2019.AIDS Behav. 2020 Oct;24(10):2797-2810. doi: 10.1007/s10461-020-02822-4. AIDS Behav. 2020. PMID: 32152815 Free PMC article.
Cited by
-
Factors Associated With Antiretroviral Adherence Among Youth Living With HIV.J Acquir Immune Defic Syndr. 2024 Mar 1;95(3):215-221. doi: 10.1097/QAI.0000000000003345. J Acquir Immune Defic Syndr. 2024. PMID: 37977178 Free PMC article.
-
A Trauma-Informed, Geospatially Aware, Just-in-Time Adaptive mHealth Intervention to Support Effective Coping Skills Among People Living With HIV in New Orleans: Development and Protocol for a Pilot Randomized Controlled Trial.JMIR Res Protoc. 2023 Oct 24;12:e47151. doi: 10.2196/47151. JMIR Res Protoc. 2023. PMID: 37874637 Free PMC article.
-
Influence of trauma on longitudinal association between depressive symptoms and use of alcohol and cannabis among young people.Psychol Trauma. 2024 Sep 26:10.1037/tra0001749. doi: 10.1037/tra0001749. Online ahead of print. Psychol Trauma. 2024. PMID: 39325418
-
Demographics of Youth With Newly Diagnosed Acute/Recent HIV Infection in Adolescent Trials Network 147: Early Treatment of Acute HIV Infection.J Adolesc Health. 2024 Mar;74(3):573-581. doi: 10.1016/j.jadohealth.2023.09.017. Epub 2023 Dec 3. J Adolesc Health. 2024. PMID: 38043041 Free PMC article.
-
Using Machine Learning to Identify Predictors of Sexually Transmitted Infections Over Time Among Young People Living With or at Risk for HIV Who Participated in ATN Protocols 147, 148, and 149.Sex Transm Dis. 2023 Nov 1;50(11):739-745. doi: 10.1097/OLQ.0000000000001854. Epub 2023 Aug 16. Sex Transm Dis. 2023. PMID: 37643402 Free PMC article.
References
-
- Centers for Disease Control and Prevention. 2018. [2018-11-23]. Estimated HIV incidence and prevalence in the United States, 2010-2015 https://www.cdc.gov/hiv/pdf/library/reports/surveillance/cdc-hiv-surveil... .
-
- Hall HI, Song R, Tang T, An Q, Prejean J, Dietz P, Hernandez AL, Green T, Harris N, McCray E, Mermin J. HIV trends in the United States: diagnoses and estimated incidence. JMIR Public Health Surveill. 2017 Feb 03;3(1):e8. doi: 10.2196/publichealth.7051. http://publichealth.jmir.org/2017/1/e8/ v3i1e8 - DOI - PMC - PubMed
-
- Allen DM, Lehman JS, Green TA, Lindegren ML, Onorato IM, Forrester W. HIV infection among homeless adults and runaway youth, United States, 1989-1992. Field Services Branch. AIDS. 1994 Nov;8(11):1593–8. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

