Prevalence and persistence of transmitted drug resistance mutations in the German HIV-1 Seroconverter Study Cohort
- PMID: 30650082
- PMCID: PMC6334938
- DOI: 10.1371/journal.pone.0209605
Prevalence and persistence of transmitted drug resistance mutations in the German HIV-1 Seroconverter Study Cohort
Abstract
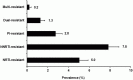
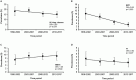
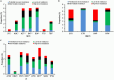
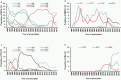
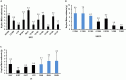
The prevalence of transmitted drug resistance (TDR) in antiretroviral therapy (ART)-naïve individuals remains stable in most developed countries despite a decrease in the prevalence of acquired drug resistance. This suggests that persistence and further transmission of HIV-1 that encodes transmitted drug resistance mutations (TDRMs) is occurring in ART-naïve individuals. In this study, we analysed the prevalence and persistence of TDRMs in the protease and reverse transcriptase-sequences of ART-naïve patients within the German HIV-1 Seroconverter Study Cohort who were infected between 1996 and 2017. The prevalence of TDRMs and baseline susceptibility to antiretroviral drugs were assessed using the Stanford HIVdb list and algorithm. Mean survival times of TDRMs were calculated by Kaplan-Meier analysis. The overall prevalence of TDR was 17.2% (95% CI 15.7-18.6, N = 466/2715). Transmitted NNRTI resistance was observed most frequently with 7.8% (95% CI 6.8-8.8), followed by NRTI resistance (5.0%, 95% CI 4.2-5.9) and PI resistance (2.8%, 95% CI 2.2-3.4). Total TDR (OR = 0.89, p = 0.034) and transmitted NRTI resistance (OR = 0.65, p = 0.000) decreased between 1996 and 2017 but has remained stable during the last decade. Viral susceptibility to NNRTIs (6.5%-6.9% for individual drugs) was mainly reduced, while <3% of the recommended NRTIs and PIs were affected. The longest mean survival times were calculated for the NNRTI mutations K103N (5.3 years, 95% CI 4.2-5.6) and E138A/G/K (8.0 years, 95% CI 5.8-10.2 / 7.9 years, 95% CI 5.4-10.3 / 6.7 years, 95% CI 6.7-6.7) and for the NRTI mutation M41L (6.4 years, 95% CI 6.0-6.7).The long persistence of single TDRMs indicates that onward transmission from ART-naïve individuals is the main cause for TDR in Germany. Transmitted NNRTI resistance was the most frequent TDR, showing simultaneously the highest impact on baseline ART susceptibility and on TDRMs with prolonged persistence. These results give cause for concern regarding the use of NNRTI in first-line regimens.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
-
- WHO. HIV/AIDS—Fact sheet 2017.
-
- Violin M, Cozzi-Lepri A, Velleca R, Vincenti A, D'Elia S, Chiodo F, et al. Risk of failure in patients with 215 HIV-1 revertants starting their first thymidine analog-containing highly active antiretroviral therapy. AIDS. 2004;18(2):227–35. . - PubMed
-
- Zaccarelli M, Tozzi V, Lorenzini P, Trotta MP, Forbici F, Visco-Comandini U, et al. Multiple drug class-wide resistance associated with poorer survival after treatment failure in a cohort of HIV-infected patients. Aids. 2005;19(10):1081–9. 10.1097/01.aids.0000174455.01369.ad PubMed PMID: WOS:000230468000014. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

