Tafenoquine versus Primaquine to Prevent Relapse of Plasmodium vivax Malaria
- PMID: 30650326
- PMCID: PMC6657225
- DOI: 10.1056/NEJMoa1802537
Tafenoquine versus Primaquine to Prevent Relapse of Plasmodium vivax Malaria
Abstract
Background: Tafenoquine, a single-dose therapy for Plasmodium vivax malaria, has been associated with relapse prevention through the clearance of P. vivax parasitemia and hypnozoites, termed "radical cure."
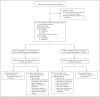
Methods: We performed a phase 3, prospective, double-blind, double-dummy, randomized, controlled trial to compare tafenoquine with primaquine in terms of safety and efficacy. The trial was conducted at seven hospitals or clinics in Peru, Brazil, Colombia, Vietnam, and Thailand and involved patients with normal glucose-6-phosphate dehydrogenase (G6PD) enzyme activity and female patients with moderate G6PD enzyme deficiency; all patients had confirmed P. vivax parasitemia. The patients were randomly assigned, in a 2:1 ratio, to receive a single 300-mg dose of tafenoquine or 15 mg of primaquine once daily for 14 days (administered under supervision); all patients received a 3-day course of chloroquine and were followed for 180 days. The primary safety outcome was a protocol-defined decrease in the hemoglobin level (>3.0 g per deciliter or ≥30% from baseline or to a level of <6.0 g per deciliter). Freedom from recurrence of P. vivax parasitemia at 6 months was the primary efficacy outcome in a planned patient-level meta-analysis of the current trial and another phase 3 trial of tafenoquine and primaquine (per-protocol populations), and an odds ratio for recurrence of 1.45 (tafenoquine vs. primaquine) was used as a noninferiority margin.
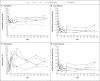
Results: A protocol-defined decrease in the hemoglobin level occurred in 4 of 166 patients (2.4%; 95% confidence interval [CI], 0.9 to 6.0) in the tafenoquine group and in 1 of 85 patients (1.2%; 95% CI, 0.2 to 6.4) in the primaquine group, for a between-group difference of 1.2 percentage points (95% CI, -4.2 to 5.0). In the patient-level meta-analysis, the percentage of patients who were free from recurrence at 6 months was 67.0% (95% CI, 61.0 to 72.3) among the 426 patients in the tafenoquine group and 72.8% (95% CI, 65.6 to 78.8) among the 214 patients in the primaquine group. The efficacy of tafenoquine was not shown to be noninferior to that of primaquine (odds ratio for recurrence, 1.81; 95% CI, 0.82 to 3.96).
Conclusions: Among patients with normal G6PD enzyme activity, the decline in hemoglobin level with tafenoquine did not differ significantly from that with primaquine. Tafenoquine showed efficacy for the radical cure of P. vivax malaria, although tafenoquine was not shown to be noninferior to primaquine. (Funded by GlaxoSmithKline and Medicines for Malaria Venture; GATHER ClinicalTrials.gov number, NCT02216123 .).
Figures

Comment in
-
Tafenoquine - A Radical Improvement?N Engl J Med. 2019 Jan 17;380(3):285-286. doi: 10.1056/NEJMe1816383. N Engl J Med. 2019. PMID: 30650321 No abstract available.
-
Tafenoquine versus Primaquine to Prevent Relapse of Plasmodium vivax Malaria.N Engl J Med. 2019 May 9;380(19):1875. doi: 10.1056/NEJMc1902327. N Engl J Med. 2019. PMID: 31067383 No abstract available.
-
Tafenoquine versus Primaquine to Prevent Relapse of Plasmodium vivax Malaria.N Engl J Med. 2019 May 9;380(19):1875-1876. doi: 10.1056/NEJMc1902327. N Engl J Med. 2019. PMID: 31067384 No abstract available.
References
-
- Control and elimination of Plasmodium vivax malaria — a technical brief. Geneva: World Health Organization, 2015. (http://www.who.int/malaria/publications/atoz/9789241509244/en/).
-
- Guidelines for the treatment of malaria. 3rd ed Geneva: World Health Organization, 2015. (http://apps.who.int/iris/bitstream/10665/162441/1/9789241549127_eng.pdf). - PubMed
-
- Llanos-Cuentas A, Lacerda MV, Rueangweerayut R, et al. . Tafenoquine plus chloroquine for the treatment and relapse prevention of Plasmodium vivax malaria (DETECTIVE): a multicentre, double-blind, randomised, phase 2b dose-selection study. Lancet 2014; 383: 1049-58. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
