Bursting at the Seams: Molecular Mechanisms Mediating Astrocyte Swelling
- PMID: 30650535
- PMCID: PMC6359623
- DOI: 10.3390/ijms20020330
Bursting at the Seams: Molecular Mechanisms Mediating Astrocyte Swelling
Abstract
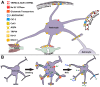
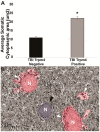
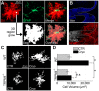
Brain swelling is one of the most robust predictors of outcome following brain injury, including ischemic, traumatic, hemorrhagic, metabolic or other injury. Depending on the specific type of insult, brain swelling can arise from the combined space-occupying effects of extravasated blood, extracellular edema fluid, cellular swelling, vascular engorgement and hydrocephalus. Of these, arguably the least well appreciated is cellular swelling. Here, we explore current knowledge regarding swelling of astrocytes, the most abundant cell type in the brain, and the one most likely to contribute to pathological brain swelling. We review the major molecular mechanisms identified to date that contribute to or mitigate astrocyte swelling via ion transport, and we touch upon the implications of astrocyte swelling in health and disease.
Keywords: Kir4.1; NKCC; Na+/K+-ATPase; SUR1-TRPM4; TRPV4; VRAC; aquaporin; astrocyte; gap junction channels; swelling.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
SUR1-TRPM4 and AQP4 form a heteromultimeric complex that amplifies ion/water osmotic coupling and drives astrocyte swelling.Glia. 2018 Jan;66(1):108-125. doi: 10.1002/glia.23231. Epub 2017 Sep 14. Glia. 2018. PMID: 28906027 Free PMC article.
-
Transient Receptor Potential Melastatin 4 Induces Astrocyte Swelling But Not Death after Diffuse Traumatic Brain Injury.J Neurotrauma. 2018 Jul 15;35(14):1694-1704. doi: 10.1089/neu.2017.5275. Epub 2018 Jun 5. J Neurotrauma. 2018. PMID: 29390943 Free PMC article.
-
Activation of NF-κB mediates astrocyte swelling and brain edema in traumatic brain injury.J Neurotrauma. 2014 Jul 15;31(14):1249-57. doi: 10.1089/neu.2013.3169. Epub 2014 May 28. J Neurotrauma. 2014. PMID: 24471369 Free PMC article.
-
Profile of intravenous glyburide for the prevention of cerebral edema following large hemispheric infarction: evidence to date.Drug Des Devel Ther. 2018 Aug 15;12:2539-2552. doi: 10.2147/DDDT.S150043. eCollection 2018. Drug Des Devel Ther. 2018. PMID: 30147301 Free PMC article. Review.
-
Cytotoxic edema: mechanisms of pathological cell swelling.Neurosurg Focus. 2007 May 15;22(5):E2. doi: 10.3171/foc.2007.22.5.3. Neurosurg Focus. 2007. PMID: 17613233 Free PMC article. Review.
Cited by
-
The role of TRPV4 in programmed cell deaths.Mol Biol Rep. 2024 Feb 1;51(1):248. doi: 10.1007/s11033-023-09199-2. Mol Biol Rep. 2024. PMID: 38300413 Review.
-
Inhibition of HMGB1 Attenuates Spinal Cord Edema by Reducing the Expression of Na+-K+-Cl- Cotransporter-1 and Na+/H+ Exchanger-1 in Both Astrocytes and Endothelial Cells After Spinal Cord Injury in Rats.J Neurotrauma. 2023 Dec;40(23-24):2522-2540. doi: 10.1089/neu.2022.0318. Epub 2023 Apr 18. J Neurotrauma. 2023. PMID: 36815608 Free PMC article.
-
Targeting Aquaporin-4 Subcellular Localization to Treat Central Nervous System Edema.Cell. 2020 May 14;181(4):784-799.e19. doi: 10.1016/j.cell.2020.03.037. Cell. 2020. PMID: 32413299 Free PMC article.
-
Somatosensory-evoked response induces extensive diffusivity and kurtosis changes associated with neural activity in rodents.Imaging Neurosci (Camb). 2025 Feb 18;3:imag_a_00445. doi: 10.1162/imag_a_00445. eCollection 2025. Imaging Neurosci (Camb). 2025. PMID: 40800919 Free PMC article.
-
Exercise Training-Related Changes in Cortical Gray Matter Diffusivity and Cognitive Function in Mild Cognitive Impairment and Healthy Older Adults.Front Aging Neurosci. 2021 Apr 8;13:645258. doi: 10.3389/fnagi.2021.645258. eCollection 2021. Front Aging Neurosci. 2021. PMID: 33897407 Free PMC article.
References
-
- Stokum J.A., Mehta R.I., Ivanova S., Yu E., Gerzanich V., Simard J.M. Heterogeneity of aquaporin-4 localization and expression after focal cerebral ischemia underlies differences in white versus grey matter swelling. Acta Neuropathol. Commun. 2015;3:1–61. doi: 10.1186/s40478-015-0239-6. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

