Expanding Repertoire of Plant Positive-Strand RNA Virus Proteases
- PMID: 30650571
- PMCID: PMC6357015
- DOI: 10.3390/v11010066
Expanding Repertoire of Plant Positive-Strand RNA Virus Proteases
Abstract
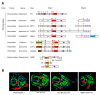
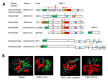
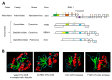
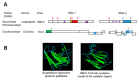
Many plant viruses express their proteins through a polyprotein strategy, requiring the acquisition of protease domains to regulate the release of functional mature proteins and/or intermediate polyproteins. Positive-strand RNA viruses constitute the vast majority of plant viruses and they are diverse in their genomic organization and protein expression strategies. Until recently, proteases encoded by positive-strand RNA viruses were described as belonging to two categories: (1) chymotrypsin-like cysteine and serine proteases and (2) papain-like cysteine protease. However, the functional characterization of plant virus cysteine and serine proteases has highlighted their diversity in terms of biological activities, cleavage site specificities, regulatory mechanisms, and three-dimensional structures. The recent discovery of a plant picorna-like virus glutamic protease with possible structural similarities with fungal and bacterial glutamic proteases also revealed new unexpected sources of protease domains. We discuss the variety of plant positive-strand RNA virus protease domains. We also highlight possible evolution scenarios of these viral proteases, including evidence for the exchange of protease domains amongst unrelated viruses.
Keywords: protease specificity; protease structure; proteolytic processing; viral proteases; virus evolution.
Conflict of interest statement
“The authors declare no conflict of interest.”
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

