Acute Exposure to Indoxyl Sulfate Impairs Endothelium-Dependent Vasorelaxation in Rat Aorta
- PMID: 30650577
- PMCID: PMC6359309
- DOI: 10.3390/ijms20020338
Acute Exposure to Indoxyl Sulfate Impairs Endothelium-Dependent Vasorelaxation in Rat Aorta
Abstract
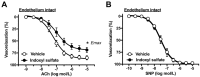
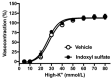
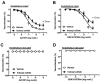
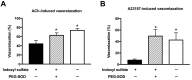
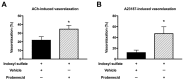
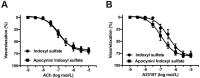
Gut microbiota are emerging as potential contributors to the regulation of host homeostasis. Dysbiosis of the gut microbiota associated with increased intestinal permeability facilitates the passage of endotoxins and other microbial products, including indoxyl sulfate in the circulation. Although an emerging body of evidence has suggested that indoxyl sulfate is a key substance for the development of chronic kidney disease, few studies have investigated the direct association of indoxyl sulfate with vascular function. We hypothesized that indoxyl sulfate adversely affects vascular function. Aortas isolated from male Wistar rat were examined in the presence or absence of indoxyl sulfate to assess the vascular function, including vasorelaxation and vasocontraction. Indoxyl sulfate (vs. vehicle) (1) decreased vasorelaxation induced by acetylcholine (ACh) but not by sodium nitroprusside; (2) had no significant alterations of noradrenaline-induced vasocontraction in the absence and presence of endothelium; (3) decreased adenylyl cyclase activator (forskolin)-induced vasorelaxation, while such a difference was eliminated by endothelial denudation; and (4) decreased vasorelaxations induced by calcium ionophore (A23187) and transient receptor potential vanilloid 4 agonist (GSK1016790A). The indoxyl sulfate-induced decrease in the vasorelaxations induced by ACh and A23187 increased by cell-permeant superoxide dismutase or by organic anion transporter inhibitor. However, apocynin, an inhibitor of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, had no effects on vasorelaxations induced by ACh, A23187, forskolin, and GSK1016790A in the presence of indoxyl sulfate. These results suggest that indoxyl sulfate directly affects the vascular function, particularly, endothelium-dependent vasorelaxation, and this effect may be attributable to increased oxidative stress after cell transportion via organic anion transporter, and such increased oxidative stress may not be attributable to activation of NADPH oxidase activation.
Keywords: Keywords: aorta; endothelial function; indoxyl sulfate; superoxide dismutase.
Conflict of interest statement
The authors declare that there are no conflict of interest.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

