Development of Natural Product-Conjugated Metal Complexes as Cancer Therapies
- PMID: 30650627
- PMCID: PMC6359354
- DOI: 10.3390/ijms20020341
Development of Natural Product-Conjugated Metal Complexes as Cancer Therapies
Abstract
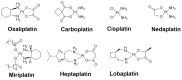
Platinum-based drugs have revolutionized cancer care, but are unfortunately associated with various adverse effects. Meanwhile, natural product scaffolds exhibit multifarious bioactivities and serve as an attractive resource for cancer therapy development. Thus, the conjugation of natural product scaffolds to metal complexes becomes an attractive strategy to reduce the severe side effects arising from the use of metal bearing drugs. This review aims to highlight the recent examples of natural product-conjugated metal complexes as cancer therapies with enhanced selectivity and efficacy. We discuss the mechanisms and features of different conjugate complexes and present an outlook and perspective for the future of this field.
Keywords: cancer therapy; cytotoxicity; natural product; transition metal complex.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

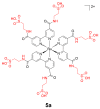
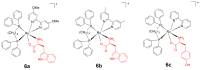

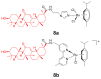
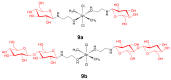

References
Publication types
MeSH terms
Substances
Grants and funding
- FRG2/16-17/007 and FRG2/17-18/003/Hong Kong Baptist University
- HMRF/14150561/Health and Medical Research Fund
- HKBU/12301115/Research Grants Council
- 21575121, 21775131, and 21628502/National Natural Science Foundation of China
- RC-IRMS/16-17/03/Hong Kong Baptist University Century Club Sponsorship Scheme 2018, the Interdisciplinary Research Matching Scheme
- RC-IRCs/17-18/03/Interdisciplinary Research Clusters Matching Scheme
- ITS/260/16FX/Innovation and Technology Fund
- C5026-16G/Collaborative Research Fund
- MPCF-001-2017/18/Matching Proof of Concept Fund
- SKLP_1718_P04/SKLEBA and HKBU Strategic Development Fund
- 0072/2018/A2/the Science and Technology Development Fund, Macao SAR
- MYRG2018-00187-ICMS and MYRG2016-00151-ICMS-QRCM/the University of Macau
LinkOut - more resources
Full Text Sources

