Function and Evolution of Nematode RNAi Pathways
- PMID: 30650636
- PMCID: PMC6468775
- DOI: 10.3390/ncrna5010008
Function and Evolution of Nematode RNAi Pathways
Abstract
Selfish genetic elements, like transposable elements or viruses, are a threat to genomic stability. A variety of processes, including small RNA-based RNA interference (RNAi)-like pathways, has evolved to counteract these elements. Amongst these, endogenous small interfering RNA and Piwi-interacting RNA (piRNA) pathways were implicated in silencing selfish genetic elements in a variety of organisms. Nematodes have several incredibly specialized, rapidly evolving endogenous RNAi-like pathways serving such purposes. Here, we review recent research regarding the RNAi-like pathways of Caenorhabditis elegans as well as those of other nematodes, to provide an evolutionary perspective. We argue that multiple nematode RNAi-like pathways share piRNA-like properties and together form a broad nematode toolkit that allows for silencing of foreign genetic elements.
Keywords: 21U RNA; 22G RNA; 26G RNA; Argonaute; C. elegans; Piwi; RdRP; nematode; piRNA; siRNA; small RNA.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
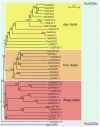
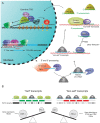
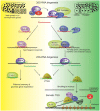

References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

