HLA-B*57:01 screening and hypersensitivity reaction to abacavir between 1999 and 2016 in the OPERA® observational database: a cohort study
- PMID: 30651100
- PMCID: PMC6334426
- DOI: 10.1186/s12981-019-0217-3
HLA-B*57:01 screening and hypersensitivity reaction to abacavir between 1999 and 2016 in the OPERA® observational database: a cohort study
Abstract
Background: HLA-B*57:01 screening was added to clinical care guidelines in 2008 to reduce the risk of hypersensitivity reaction from abacavir. The uptake of HLA-B*57:01 screening and incidence of hypersensitivity reaction were assessed in a prospective clinical cohort in the United States to evaluate the effectiveness of this intervention.
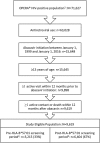
Methods: We included all patients initiating an abacavir-containing regimen for the first time in the pre-HLA-B*57:01 screening period (January 1, 1999 to June 14, 2008) or the post-HLA-B*57:01 screening period (June 15, 2008 to January 1, 2016). Yearly incidence of both HLA-B*57:01 screening and physician panel-adjudicated hypersensitivity reactions were calculated and compared.
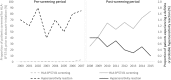
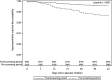
Results: Of the 9619 patients eligible for the study, 33% initiated abacavir in the pre-screening period and 67% in the post-screening period. Incidence of HLA-B*57:01 screening prior to abacavir initiation increased from 43% in 2009 to 84% in 2015. The incidence of definite or probable hypersensitivity reactions decreased from 1.3% in the pre-screening period to 0.8% in 2009 and further to 0.2% in 2015 in the post-screening period.
Conclusions: Frequency of HLA-B*57:01 screening increased steadily since its first inclusion in treatment guidelines in the United States. This increase in screening was accompanied by a decreasing incidence of definite or probable hypersensitivity reactions over the same period. However, a considerable proportion of patients initiating abacavir were not screened, representing a failed opportunity to prevent hypersensitivity reactions. Where HLA-B*57:01 screening is standard of care, patients should be confirmed negative for this allele before starting abacavir treatment.
Keywords: Abacavir; Cohort; HIV; HLA-B*57:01 screening; Hypersensitivity reaction.
Figures
Similar articles
-
Abacavir Hypersensitivity Reaction Reporting Rates During a Decade of HLA-B*5701 Screening as a Risk-Mitigation Measure.Pharmacotherapy. 2019 Jan;39(1):40-54. doi: 10.1002/phar.2196. Epub 2018 Dec 11. Pharmacotherapy. 2019. PMID: 30414209 Free PMC article.
-
Abacavir adverse reactions related with HLA-B*57: 01 haplotype in a large cohort of patients infected with HIV.Pharmacogenet Genomics. 2020 Oct;30(8):167-174. doi: 10.1097/FPC.0000000000000409. Pharmacogenet Genomics. 2020. PMID: 32453265
-
HLA-B*5701 screening for hypersensitivity to abacavir.N Engl J Med. 2008 Feb 7;358(6):568-79. doi: 10.1056/NEJMoa0706135. N Engl J Med. 2008. PMID: 18256392 Clinical Trial.
-
Studies on abacavir-induced hypersensitivity reaction: a successful example of translation of pharmacogenetics to personalized medicine.Sci China Life Sci. 2013 Feb;56(2):119-24. doi: 10.1007/s11427-013-4438-8. Epub 2013 Feb 8. Sci China Life Sci. 2013. PMID: 23393027 Review.
-
Abacavir pharmacogenetics--from initial reports to standard of care.Pharmacotherapy. 2013 Jul;33(7):765-75. doi: 10.1002/phar.1278. Epub 2013 May 3. Pharmacotherapy. 2013. PMID: 23649914 Free PMC article. Review.
Cited by
-
Tools for Etiologic Diagnosis of Drug-Induced Allergic Conditions.Int J Mol Sci. 2023 Aug 8;24(16):12577. doi: 10.3390/ijms241612577. Int J Mol Sci. 2023. PMID: 37628756 Free PMC article. Review.
-
Critical Review of Gaps in the Diagnosis and Management of Drug-Induced Liver Injury Associated with Severe Cutaneous Adverse Reactions.J Clin Med. 2021 Nov 15;10(22):5317. doi: 10.3390/jcm10225317. J Clin Med. 2021. PMID: 34830594 Free PMC article. Review.
-
A Comprehensive Review of HLA and Severe Cutaneous Adverse Drug Reactions: Implication for Clinical Pharmacogenomics and Precision Medicine.Pharmaceuticals (Basel). 2021 Oct 25;14(11):1077. doi: 10.3390/ph14111077. Pharmaceuticals (Basel). 2021. PMID: 34832859 Free PMC article. Review.
-
Genotyping for HLA Risk Alleles to Prevent Drug Hypersensitivity Reactions: Impact Analysis.Pharmaceuticals (Basel). 2021 Dec 21;15(1):4. doi: 10.3390/ph15010004. Pharmaceuticals (Basel). 2021. PMID: 35056062 Free PMC article.
-
Experimental pharmacology in precision medicine.Pharmacol Res Perspect. 2023 Dec;11(6):e01147. doi: 10.1002/prp2.1147. Pharmacol Res Perspect. 2023. PMID: 37885364 Free PMC article. No abstract available.
References
-
- McDowell JA, Lou Y, Symonds WS, Stein DS. Multiple-dose pharmacokinetics and pharmacodynamics of abacavir alone and in combination with zidovudine in human immunodeficiency virus-infected adults. Antimicrob Agents Chemother. 2000;44(8):2061–2067. doi: 10.1128/AAC.44.8.2061-2067.2000. - DOI - PMC - PubMed
-
- Weller S, Radomski KM, Lou Y, Stein DS. Population pharmacokinetics and pharmacodynamic modeling of abacavir (1592U89) from a dose-ranging, double-blind, randomized monotherapy trial with human immunodeficiency virus-infected subjects. Antimicrob Agents Chemother. 2000;44(8):2052–2060. doi: 10.1128/AAC.44.8.2052-2060.2000. - DOI - PMC - PubMed
-
- Wang LH, Chittick GE, McDowell JA. Single-dose pharmacokinetics and safety of abacavir (1592U89), zidovudine, and lamivudine administered alone and in combination in adults with human immunodeficiency virus infection. Antimicrob Agents Chemother. 1999;43(7):1708–1715. doi: 10.1128/AAC.43.7.1708. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

