HLA-B*57:01 screening and hypersensitivity reaction to abacavir between 1999 and 2016 in the OPERA® observational database: a cohort study
- PMID: 30651100
- PMCID: PMC6334426
- DOI: 10.1186/s12981-019-0217-3
HLA-B*57:01 screening and hypersensitivity reaction to abacavir between 1999 and 2016 in the OPERA® observational database: a cohort study
Abstract
Background: HLA-B*57:01 screening was added to clinical care guidelines in 2008 to reduce the risk of hypersensitivity reaction from abacavir. The uptake of HLA-B*57:01 screening and incidence of hypersensitivity reaction were assessed in a prospective clinical cohort in the United States to evaluate the effectiveness of this intervention.
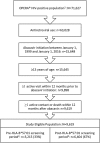
Methods: We included all patients initiating an abacavir-containing regimen for the first time in the pre-HLA-B*57:01 screening period (January 1, 1999 to June 14, 2008) or the post-HLA-B*57:01 screening period (June 15, 2008 to January 1, 2016). Yearly incidence of both HLA-B*57:01 screening and physician panel-adjudicated hypersensitivity reactions were calculated and compared.
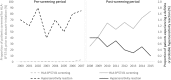
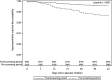
Results: Of the 9619 patients eligible for the study, 33% initiated abacavir in the pre-screening period and 67% in the post-screening period. Incidence of HLA-B*57:01 screening prior to abacavir initiation increased from 43% in 2009 to 84% in 2015. The incidence of definite or probable hypersensitivity reactions decreased from 1.3% in the pre-screening period to 0.8% in 2009 and further to 0.2% in 2015 in the post-screening period.
Conclusions: Frequency of HLA-B*57:01 screening increased steadily since its first inclusion in treatment guidelines in the United States. This increase in screening was accompanied by a decreasing incidence of definite or probable hypersensitivity reactions over the same period. However, a considerable proportion of patients initiating abacavir were not screened, representing a failed opportunity to prevent hypersensitivity reactions. Where HLA-B*57:01 screening is standard of care, patients should be confirmed negative for this allele before starting abacavir treatment.
Keywords: Abacavir; Cohort; HIV; HLA-B*57:01 screening; Hypersensitivity reaction.
Figures
References
-
- McDowell JA, Lou Y, Symonds WS, Stein DS. Multiple-dose pharmacokinetics and pharmacodynamics of abacavir alone and in combination with zidovudine in human immunodeficiency virus-infected adults. Antimicrob Agents Chemother. 2000;44(8):2061–2067. doi: 10.1128/AAC.44.8.2061-2067.2000. - DOI - PMC - PubMed
-
- Weller S, Radomski KM, Lou Y, Stein DS. Population pharmacokinetics and pharmacodynamic modeling of abacavir (1592U89) from a dose-ranging, double-blind, randomized monotherapy trial with human immunodeficiency virus-infected subjects. Antimicrob Agents Chemother. 2000;44(8):2052–2060. doi: 10.1128/AAC.44.8.2052-2060.2000. - DOI - PMC - PubMed
-
- Wang LH, Chittick GE, McDowell JA. Single-dose pharmacokinetics and safety of abacavir (1592U89), zidovudine, and lamivudine administered alone and in combination in adults with human immunodeficiency virus infection. Antimicrob Agents Chemother. 1999;43(7):1708–1715. doi: 10.1128/AAC.43.7.1708. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

