Hydrodynamic shear stress promotes epithelial-mesenchymal transition by downregulating ERK and GSK3β activities
- PMID: 30651129
- PMCID: PMC6335853
- DOI: 10.1186/s13058-018-1071-2
Hydrodynamic shear stress promotes epithelial-mesenchymal transition by downregulating ERK and GSK3β activities
Abstract
Background: Epithelial-mesenchymal transition (EMT) occurs in the tumor microenvironment and presents an important mechanism of tumor cell intravasation, stemness acquisition, and metastasis. During metastasis, tumor cells enter the circulation to gain access to distant tissues, but how this fluid microenvironment influences cancer cell biology is poorly understood.
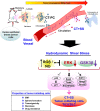
Methods and results: Here, we present both in vivo and in vitro evidence that EMT-like transition also occurs in circulating tumor cells (CTCs) as a result of hydrodynamic shear stress (+SS), which promotes conversion of CD24middle/CD44high/CD133middle/CXCR4low/ALDH1low primary patient epithelial tumor cells into specific high sphere-forming CD24low/CD44low/CD133high/CXCR4high/ALDH1high cancer stem-like cells (CSLCs) or tumor-initiating cells (TICs) with elevated tumor progression and metastasis capacity in vitro and in vivo. We demonstrate that conversion of CSLCs/TICs from epithelial tumor cells via +SS is dependent on reactive oxygen species (ROS)/nitric oxide (NO) generation, and suppression of extracellular signal-related kinase (ERK)/glycogen synthase kinase (GSK)3β, a mechanism similar to that operating in embryonic stem cells to prevent their differentiation while promoting self-renewal.
Conclusion: Fluid shear stress experienced during systemic circulation of human breast tumor cells can lead to specific acquisition of mesenchymal stem cell (MSC)-like potential that promotes EMT, mesenchymal-epithelial transition, and metastasis to distant organs. Our data revealed that biomechanical forces appeared to be important microenvironmental factors that not only drive hematopoietic development but also lead to acquisition of CSLCs/TIC potential in cancer metastasis. Our data highlight that +SS is a critical factor that promotes the conversion of CTCs into distinct TICs in blood circulation by endowing plasticity to these cells and by maintaining their self-renewal signaling pathways.
Keywords: EMT/MET; ERK-GSK3β; Hydrodynamic shear stress; ROS/NO; Tumor-initiating cells.
Conflict of interest statement
Ethics approval
All animal experiments were performed in accordance with protocols reviewed and approved by Institutional Animal Care and Use Committee (IACUC) of Konkuk University.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures





Similar articles
-
MCP-1-induced ERK/GSK-3β/Snail signaling facilitates the epithelial-mesenchymal transition and promotes the migration of MCF-7 human breast carcinoma cells.Cell Mol Immunol. 2017 Jul;14(7):621-630. doi: 10.1038/cmi.2015.106. Epub 2016 Mar 21. Cell Mol Immunol. 2017. PMID: 26996066 Free PMC article.
-
Fluid Shear Stress Induces EMT of Circulating Tumor Cells via JNK Signaling in Favor of Their Survival during Hematogenous Dissemination.Int J Mol Sci. 2020 Oct 30;21(21):8115. doi: 10.3390/ijms21218115. Int J Mol Sci. 2020. PMID: 33143160 Free PMC article.
-
Expression of Six1 in luminal breast cancers predicts poor prognosis and promotes increases in tumor initiating cells by activation of extracellular signal-regulated kinase and transforming growth factor-beta signaling pathways.Breast Cancer Res. 2012 Jul 5;14(4):R100. doi: 10.1186/bcr3219. Breast Cancer Res. 2012. PMID: 22765220 Free PMC article.
-
Breast cancer stem cells: Multiple capacities in tumor metastasis.Cancer Lett. 2014 Jul 10;349(1):1-7. doi: 10.1016/j.canlet.2014.03.036. Epub 2014 Apr 12. Cancer Lett. 2014. PMID: 24727284 Free PMC article. Review.
-
Nitric oxide and interactions with reactive oxygen species in the development of melanoma, breast, and colon cancer: A redox signaling perspective.Nitric Oxide. 2019 Aug 1;89:1-13. doi: 10.1016/j.niox.2019.04.009. Epub 2019 Apr 19. Nitric Oxide. 2019. PMID: 31009708 Review.
Cited by
-
ECM-transmitted shear stress induces apoptotic cell extrusion in early breast gland development.Front Cell Dev Biol. 2022 Aug 29;10:947430. doi: 10.3389/fcell.2022.947430. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36105352 Free PMC article.
-
Matrix stiffness induces epithelial-to-mesenchymal transition via Piezo1-regulated calcium flux in prostate cancer cells.iScience. 2023 Feb 25;26(4):106275. doi: 10.1016/j.isci.2023.106275. eCollection 2023 Apr 21. iScience. 2023. PMID: 36950111 Free PMC article.
-
An integrated approach to understand fluid shear stress-driven and reactive oxygen species-mediated metastasis of colon adenocarcinoma through mRNA-miRNA-lncRNA-circRNA networks.Mol Genet Genomics. 2022 Sep;297(5):1353-1370. doi: 10.1007/s00438-022-01924-z. Epub 2022 Jul 13. Mol Genet Genomics. 2022. PMID: 35831469
-
Mechanobiology and survival strategies of circulating tumor cells: a process towards the invasive and metastatic phenotype.Front Cell Dev Biol. 2023 May 5;11:1188499. doi: 10.3389/fcell.2023.1188499. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 37215087 Free PMC article. Review.
-
Circulating Tumor Microenvironment in Metastasis.Cancer Res. 2025 Apr 15;85(8):1354-1367. doi: 10.1158/0008-5472.CAN-24-1241. Cancer Res. 2025. PMID: 39992721 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

