Delamanid, linezolid, levofloxacin, and pyrazinamide for the treatment of patients with fluoroquinolone-sensitive multidrug-resistant tuberculosis (Treatment Shortening of MDR-TB Using Existing and New Drugs, MDR-END): study protocol for a phase II/III, multicenter, randomized, open-label clinical trial
- PMID: 30651149
- PMCID: PMC6335682
- DOI: 10.1186/s13063-018-3053-1
Delamanid, linezolid, levofloxacin, and pyrazinamide for the treatment of patients with fluoroquinolone-sensitive multidrug-resistant tuberculosis (Treatment Shortening of MDR-TB Using Existing and New Drugs, MDR-END): study protocol for a phase II/III, multicenter, randomized, open-label clinical trial
Abstract
Background: Treatment success rates of multidrug-resistant tuberculosis (MDR-TB) remain unsatisfactory, and long-term use of second-line anti-TB drugs is accompanied by the frequent occurrence of adverse events, low treatment compliance, and high costs. The development of new efficient regimens with shorter treatment durations for MDR-TB will solve these issues and improve treatment outcomes.
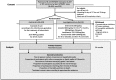
Methods: This study is a phase II/III, multicenter, randomized, open-label clinical trial of non-inferiority design comparing a new regimen to the World Health Organization-endorsed conventional regimen for fluoroquinolone-sensitive MDR-TB. The control arm uses a conventional treatment regimen with second-line drugs including injectables for 20-24 months. The investigational arm uses a new shorter regimen including delamanid, linezolid, levofloxacin, and pyrazinamide for 9 or 12 months depending on time to sputum culture conversion. The primary outcome is the treatment success rate at 24 months after treatment initiation. Secondary outcomes include time to sputum culture conversion on liquid and solid media, proportions of sputum culture conversion on liquid media after 2 and 6 months of treatment, treatment success rate according to pyrazinamide resistance, and occurrence of adverse events grade 3 and above as evaluated by the Common Terminology Criteria for Adverse Events. Based on an α = 0.025 level of significance (one-sided test), a power of 80%, and a < 10% difference in treatment success rate between the control and investigational arms (80% vs. 70%) when the anticipated actual success rate in the treatment group is assumed to be 90%, the number of participants needed per arm to show non-inferiority of the investigational regimen was calculated as 48. Additionally, assuming the proportion of fluoroquinolone-susceptible MDR-TB among participants as 50%, and 5% loss to follow-up, the number of participants is calculated as N/( 0.50 × 0.95), resulting in 102 persons per group (204 in total).
Discussion: This trial will reveal the effectiveness and safety of a new shorter regimen comprising four oral drugs, including delamanid, linezolid, levofloxacin, and pyrazinamide, for the treatment of fluoroquinolone-sensitive MDR-TB. Results from this trial will provide evidence for adopting a shorter and more convenient treatment regimen for MDR-TB.
Trial registration: ClincalTrials.gov, NCT02619994 . Registered on 2 December 2015.
Keywords: Delamanid; Linezolid; Multicenter randomized trial; Multidrug-resistant tuberculosis; Non-inferiority; Shorter regimen; Tuberculosis.
Conflict of interest statement
Ethics approval and consent to participate
The trial received ethical approval from the Institutional Review Boards of all participating sites: Seoul National University Hospital (approval number H-1508-105-696), National Medical Center (approval number H-1602-063-002), Dankook University Hospital (approval number DKUH 2016-03-001), Pusan National University Hospital (approval number H-1602-010-053), Korea University Ansan Hospital (approval number AS16181), Samsung Medical Center (approval number SMC 2016-02-122), Asan Medical Center (approval number 2016-0316), Seoul Metropolitan Government (SMG)-Seoul National University (SNU) Boramae Medical Center (approval number 26-2016-22), Pusan National University Yangsan Hospital (approval number 04-2016-003), Severance Hospital, Yonsei University College of Medicine (approval number 4-2016-0141), Ulsan University Hospital (approval number UUH 2016-02-013), and the Catholic University of Korea Incheon St. Mary’s Hospital (approval number OC16MNMS0020). Participants must provide signed and dated written informed consent prior to undergoing any study-specific procedures.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
-
- World Health Organization . Global tuberculosis report 2014. 2014.
-
- World Health Organization. Global tuberculosis report 2017. 2017
-
- Korea Centers for Disease Control and Prevention . Annual report on the notified tuberculosis in Korea 2016. Cheongju: Korea Centers for Disease Control and Prevention; 2016. p. 44.
-
- World Health Organization . Treatment guidelines for drug-resistant tuberculosis. 2016.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical