Phage Therapy in the Postantibiotic Era
- PMID: 30651225
- PMCID: PMC6431132
- DOI: 10.1128/CMR.00066-18
Phage Therapy in the Postantibiotic Era
Abstract
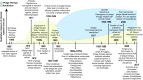
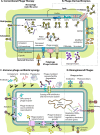
Antibiotic resistance is arguably the biggest current threat to global health. An increasing number of infections are becoming harder or almost impossible to treat, carrying high morbidity, mortality, and financial cost. The therapeutic use of bacteriophages, viruses that infect and kill bacteria, is well suited to be part of the multidimensional strategies to combat antibiotic resistance. Although phage therapy was first implemented almost a century ago, it was brought to a standstill after the successful introduction of antibiotics. Now, with the rise of antibiotic resistance, phage therapy is experiencing a well-deserved rebirth. Among the admittedly vast literature recently published on this topic, this review aims to provide a forward-looking perspective on phage therapy and its role in modern society. We cover the key points of the antibiotic resistance crisis and then explain the biological and evolutionary principles that support the use of phages, their interaction with the immune system, and a comparison with antibiotic therapy. By going through up-to-date reports and, whenever possible, human clinical trials, we examine the versatility of phage therapy. We discuss conventional approaches as well as novel strategies, including the use of phage-antibiotic combinations, phage-derived enzymes, exploitation of phage resistance mechanisms, and phage bioengineering. Finally, we discuss the benefits of phage therapy beyond the clinical perspective, including opportunities for scientific outreach and effective education, interdisciplinary collaboration, cultural and economic growth, and even innovative use of social media, making the case that phage therapy is more than just an alternative to antibiotics.
Keywords: antibiotic resistance; bacteriophage therapy; bacteriophages.
Copyright © 2019 American Society for Microbiology.
Figures
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

