The Role of Plastidic Trigger Factor Serving Protein Biogenesis in Green Algae and Land Plants
- PMID: 30651302
- PMCID: PMC6393800
- DOI: 10.1104/pp.18.01252
The Role of Plastidic Trigger Factor Serving Protein Biogenesis in Green Algae and Land Plants
Abstract
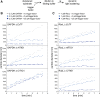
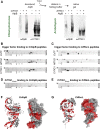
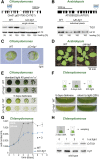
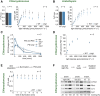
Biochemical processes in chloroplasts are important for virtually all life forms. Tight regulation of protein homeostasis and the coordinated assembly of protein complexes, composed of both imported and locally synthesized subunits, are vital to plastid functionality. Protein biogenesis requires the action of cotranslationally acting molecular chaperones. One such chaperone is trigger factor (TF), which is known to cotranslationally bind most newly synthesized proteins in bacteria, thereby assisting their correct folding and maturation. However, how these processes are regulated in chloroplasts remains poorly understood. We report here functional investigation of chloroplast-localized TF (TIG1) in the green alga (Chlamydomonas reinhardtii) and the vascular land plant Arabidopsis (Arabidopsis thaliana). We show that chloroplastic TIG1 evolved as a specialized chaperone. Unlike other plastidic chaperones that are functionally interchangeable with their prokaryotic counterpart, TIG1 was not able to complement the broadly acting ortholog in Escherichia coli. Whereas general chaperone properties such as the prevention of aggregates or substrate recognition seems to be conserved between bacterial and plastidic TFs, plant TIG1s differed by associating with only a relatively small population of translating ribosomes. Furthermore, a reduction of plastidic TIG1 levels leads to deregulated protein biogenesis at the expense of increased translation, thereby disrupting the chloroplast energy household. This suggests a central role of TIG1 in protein biogenesis in the chloroplast.
© 2019 American Society of Plant Biologists. All Rights Reserved.
Figures


References
-
- Agashe VR, Guha S, Chang H-C, Genevaux P, Hayer-Hartl M, Stemp M, Georgopoulos C, Hartl FU, Barral JM (2004) Function of trigger factor and DnaK in multidomain protein folding: increase in yield at the expense of folding speed. Cell 117: 199–209 - PubMed
-
- Allen JF, de Paula WB, Puthiyaveetil S, Nield J (2011) A structural phylogenetic map for chloroplast photosynthesis. Trends Plant Sci 16: 645–655 - PubMed
-
- Alonso JM, Stepanova AN, Leisse TJ, Kim CJ, Chen H, Shinn P, Stevenson DK, Zimmerman J, Barajas P, Cheuk R, et al. (2003) Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301: 653–657 - PubMed
-
- Bai C, Guo P, Zhao Q, Lv Z, Zhang S, Gao F, Gao L, Wang Y, Tian Z, Wang J, et al. (2015) Protomer roles in chloroplast chaperonin assembly and function. Mol Plant 8: 1478–1492 - PubMed
-
- Bailleul B, Cardol P, Breyton C, Finazzi G (2010) Electrochromism: A useful probe to study algal photosynthesis. Photosynth Res 106: 179–189 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

