In vivo measurement of trabecular meshwork stiffness in a corticosteroid-induced ocular hypertensive mouse model
- PMID: 30651311
- PMCID: PMC6358695
- DOI: 10.1073/pnas.1814889116
In vivo measurement of trabecular meshwork stiffness in a corticosteroid-induced ocular hypertensive mouse model
Abstract
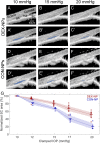
Ocular corticosteroids are commonly used clinically. Unfortunately, their administration frequently leads to ocular hypertension, i.e., elevated intraocular pressure (IOP), which, in turn, can progress to a form of glaucoma known as steroid-induced glaucoma. The pathophysiology of this condition is poorly understood yet shares similarities with the most common form of glaucoma. Using nanotechnology, we created a mouse model of corticosteroid-induced ocular hypertension. This model functionally and morphologically resembles human ocular hypertension, having titratable, robust, and sustained IOPs caused by increased resistance to aqueous humor outflow. Using this model, we then interrogated the biomechanical properties of the trabecular meshwork (TM), including the inner wall of Schlemm's canal (SC), tissues known to strongly influence IOP and to be altered in other forms of glaucoma. Specifically, using spectral domain optical coherence tomography, we observed that SC in corticosteroid-treated mice was more resistant to collapse at elevated IOPs, reflecting increased TM stiffness determined by inverse finite element modeling. Our noninvasive approach to monitoring TM stiffness in vivo is applicable to other forms of glaucoma and has significant potential to monitor TM function and thus positively affect the clinical care of glaucoma, the leading cause of irreversible blindness worldwide.
Keywords: Schlemm’s canal; finite element modeling; glaucoma; nanoparticle; optical coherence tomography.
Copyright © 2019 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Ciulla TA, Walker JD, Fong DS, Criswell MH. Corticosteroids in posterior segment disease: An update on new delivery systems and new indications. Curr Opin Ophthalmol. 2004;15:211–220. - PubMed
-
- Al-Khersan H, Hariprasad SM, Chhablani J. Dex Implant Study Group Early response to intravitreal dexamethasone implant therapy in diabetic macular edema may predict visual outcome. Am J Ophthalmol. 2017;184:121–128. - PubMed
-
- Miller K, Fortun JA. Diabetic macular edema: Current understanding, pharmacologic treatment options, and developing therapies. Asia Pac J Ophthalmol (Phila) 2018;7:28–35. - PubMed
-
- Narayanan R, Kuppermann BD. Corticosteroids and anti-complement therapy in retinal diseases. Handb Exp Pharmacol. 2017;242:309–320. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

