Amplification and Suppression of Traveling Waves along the Mouse Organ of Corti: Evidence for Spatial Variation in the Longitudinal Coupling of Outer Hair Cell-Generated Forces
- PMID: 30651330
- PMCID: PMC6407303
- DOI: 10.1523/JNEUROSCI.2608-18.2019
Amplification and Suppression of Traveling Waves along the Mouse Organ of Corti: Evidence for Spatial Variation in the Longitudinal Coupling of Outer Hair Cell-Generated Forces
Abstract
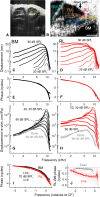
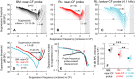
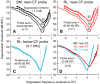
Mammalian hearing sensitivity and frequency selectivity depend on a mechanical amplification process mediated by outer hair cells (OHCs). OHCs are situated within the organ of Corti atop the basilar membrane (BM), which supports sound-evoked traveling waves. It is well established that OHCs generate force to selectively amplify BM traveling waves where they peak, and that amplification accumulates from one location to the next over this narrow cochlear region. However, recent measurements demonstrate that traveling waves along the apical surface of the organ of Corti, the reticular lamina (RL), are amplified over a much broader region. Whether OHC forces accumulate along the length of the RL traveling wave to provide a form of "global" cochlear amplification is unclear. Here we examined the spatial accumulation of RL amplification. In mice of either sex, we used tones to suppress amplification from different cochlear regions and examined the effect on RL vibrations near and far from the traveling-wave peak. We found that although OHC forces amplify the entire RL traveling wave, amplification only accumulates near the peak, over the same region where BM motion is amplified. This contradicts the notion that RL motion is involved in a global amplification mechanism and reveals that the mechanical properties of the BM and organ of Corti tune how OHC forces accumulate spatially. Restricting the spatial buildup of amplification enhances frequency selectivity by sharpening the peaks of cochlear traveling waves and constrains the number of OHCs responsible for mechanical sensitivity at each location.SIGNIFICANCE STATEMENT Outer hair cells generate force to amplify traveling waves within the mammalian cochlea. This force generation is critical to the ability to detect and discriminate sounds. Nevertheless, how these forces couple to the motions of the surrounding structures and integrate along the cochlear length remains poorly understood. Here we demonstrate that outer hair cell-generated forces amplify traveling-wave motion on the organ of Corti throughout the wave's extent, but that these forces only accumulate longitudinally over a region near the wave's peak. The longitudinal coupling of outer hair cell-generated forces is therefore spatially tuned, likely by the mechanical properties of the basilar membrane and organ of Corti. Our findings provide new insight into the mechanical processes that underlie sensitive hearing.
Keywords: basilar membrane; cochlear amplification; reticular lamina; traveling wave.
Copyright © 2019 the authors 0270-6474/19/391805-12$15.00/0.
Figures



Similar articles
-
Two-Dimensional Cochlear Micromechanics Measured In Vivo Demonstrate Radial Tuning within the Mouse Organ of Corti.J Neurosci. 2016 Aug 3;36(31):8160-73. doi: 10.1523/JNEUROSCI.1157-16.2016. J Neurosci. 2016. PMID: 27488636 Free PMC article.
-
The Reduced Cortilymph Flow Path in the Short-Wave Region Allows Outer Hair Cells to Produce Focused Traveling-Wave Amplification.J Assoc Res Otolaryngol. 2025 Feb;26(1):49-61. doi: 10.1007/s10162-025-00976-3. Epub 2025 Feb 7. J Assoc Res Otolaryngol. 2025. PMID: 39920422
-
The cortilymph wave: Its relation to the traveling wave, auditory-nerve responses, and low-frequency downward glides.Hear Res. 2025 Jun;462:109279. doi: 10.1016/j.heares.2025.109279. Epub 2025 Apr 16. Hear Res. 2025. PMID: 40253777 Review.
-
Corti Fluid Is a Medium for Outer Hair Cell Force Transmission.J Neurosci. 2025 Jan 15;45(3):e1033242024. doi: 10.1523/JNEUROSCI.1033-24.2024. J Neurosci. 2025. PMID: 39496490 Free PMC article.
-
The interplay of organ-of-Corti vibrational modes, not tectorial- membrane resonance, sets outer-hair-cell stereocilia phase to produce cochlear amplification.Hear Res. 2020 Sep 15;395:108040. doi: 10.1016/j.heares.2020.108040. Epub 2020 Jul 30. Hear Res. 2020. PMID: 32784038 Free PMC article. Review.
Cited by
-
Interactions between Passive and Active Vibrations in the Organ of Corti In Vitro.Biophys J. 2020 Jul 21;119(2):314-325. doi: 10.1016/j.bpj.2020.06.011. Epub 2020 Jun 17. Biophys J. 2020. PMID: 32579963 Free PMC article.
-
The origin of mechanical harmonic distortion within the organ of Corti in living gerbil cochleae.Commun Biol. 2021 Aug 25;4(1):1008. doi: 10.1038/s42003-021-02540-0. Commun Biol. 2021. PMID: 34433876 Free PMC article.
-
Visualizing motions within the cochlea's organ of Corti and illuminating cochlear mechanics with optical coherence tomography.Hear Res. 2025 Jan;455:109154. doi: 10.1016/j.heares.2024.109154. Epub 2024 Nov 27. Hear Res. 2025. PMID: 39626338 Review.
-
Low-side and multitone suppression in the base of the gerbil cochlea.Biophys J. 2025 Jan 21;124(2):297-315. doi: 10.1016/j.bpj.2024.12.004. Epub 2024 Dec 4. Biophys J. 2025. PMID: 39639771
-
Optical coherence tomography imaging demonstrates endolymphatic hydrops in the lateral and posterior semicircular canals in noise-exposed mice.Hear Res. 2025 Jul 29;466:109380. doi: 10.1016/j.heares.2025.109380. Online ahead of print. Hear Res. 2025. PMID: 40752142
References
-
- Ashmore J, Avan P, Brownell WE, Dallos P, Dierkes K, Fettiplace R, Grosh K, Hackney CM, Hudspeth AJ, Jülicher F, Lindner B, Martin P, Meaud J, Petit C, Santos-Sacchi JR, Sacchi JR, Canlon B (2010) The remarkable cochlear amplifier. Hear Res 266:1–17. 10.1016/j.heares.2010.05.001 - DOI - PMC - PubMed
-
- Békésy von G. (1960) Experiments in hearing. New York: McGraw-Hill.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
