Angiogenic Host Defense Peptide AG-30/5C and Bradykinin B2 Receptor Antagonist Icatibant Are G Protein Biased Agonists for MRGPRX2 in Mast Cells
- PMID: 30651343
- PMCID: PMC6369923
- DOI: 10.4049/jimmunol.1801227
Angiogenic Host Defense Peptide AG-30/5C and Bradykinin B2 Receptor Antagonist Icatibant Are G Protein Biased Agonists for MRGPRX2 in Mast Cells
Abstract
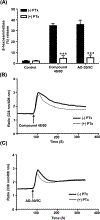
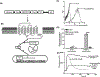
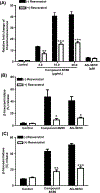
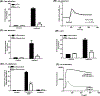
AG-30/5C is an angiogenic host defense peptide that activates human mast cells (MC) via an unknown mechanism. Using short hairpin RNA-silenced human MC line LAD2 and stably transfected RBL-2H3 cells, we demonstrate that AG-30/5C induces MC degranulation via Mas-related G protein-coupled receptor X2 (MRGPRX2). Most G protein-coupled receptors signal via parallel and independent pathways mediated by G proteins and β-arrestins. AG-30/5C and compound 48/80 induced similar maximal MC degranulation via MRGPRX2, which was abolished by pertussis toxin. However, compound 48/80 induced a robust β-arrestin activation as determined by transcriptional activation following arrestin translocation (Tango), but AG-30/5C did not. Overnight culture of MC with compound 48/80 resulted in reduced cell surface MRGPRX2 expression, and this was associated with a significant decrease in subsequent MC degranulation in response to compound 48/80 or AG-30/5C. However, AG-30/5C pretreatment had no effect on cell surface MRGPRX2 expression or degranulation in response to compound 48/80 or AG-30/5C. Icatibant, a bradykinin B2 receptor antagonist, promotes MC degranulation via MRGPRX2 and causes pseudoallergic drug reaction. Icatibant caused MC degranulation via a pertussis toxin-sensitive G protein but did not activate β-arrestin. A screen of the National Institutes of Health Clinical Collection library led to the identification of resveratrol as an inhibitor of MRGPRX2. Resveratrol inhibited compound 48/80-induced Tango and MC degranulation in response to compound 48/80, AG-30/5C, and Icatibant. This study demonstrates the novel finding that AG-30/5C and Icatibant serve as G protein-biased agonists for MRGPRX2, but compound 48/80 signals via both G protein and β-arrestin with distinct differences in receptor regulation.
Copyright © 2019 by The American Association of Immunologists, Inc.
Figures
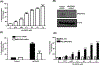


Similar articles
-
Murepavadin, a Small Molecule Host Defense Peptide Mimetic, Activates Mast Cells via MRGPRX2 and MrgprB2.Front Immunol. 2021 Jun 23;12:689410. doi: 10.3389/fimmu.2021.689410. eCollection 2021. Front Immunol. 2021. PMID: 34248979 Free PMC article.
-
Naturally Occurring Missense MRGPRX2 Variants Display Loss of Function Phenotype for Mast Cell Degranulation in Response to Substance P, Hemokinin-1, Human β-Defensin-3, and Icatibant.J Immunol. 2018 Jul 15;201(2):343-349. doi: 10.4049/jimmunol.1701793. Epub 2018 May 23. J Immunol. 2018. PMID: 29794017 Free PMC article.
-
Inhibition of MRGPRX2 but not FcεRI or MrgprB2-mediated mast cell degranulation by a small molecule inverse receptor agonist.Front Immunol. 2022 Oct 6;13:1033794. doi: 10.3389/fimmu.2022.1033794. eCollection 2022. Front Immunol. 2022. PMID: 36275683 Free PMC article.
-
Mast cell degranulation and bradykinin-induced angioedema - searching for the missing link.Front Immunol. 2024 May 15;15:1399459. doi: 10.3389/fimmu.2024.1399459. eCollection 2024. Front Immunol. 2024. PMID: 38812508 Free PMC article. Review.
-
Unlocking the Non-IgE-Mediated Pseudo-Allergic Reaction Puzzle with Mas-Related G-Protein Coupled Receptor Member X2 (MRGPRX2).Cells. 2021 Apr 27;10(5):1033. doi: 10.3390/cells10051033. Cells. 2021. PMID: 33925682 Free PMC article. Review.
Cited by
-
MRGPRX2-Mediated Degranulation of Human Skin Mast Cells Requires the Operation of Gαi, Gαq, Ca++ Channels, ERK1/2 and PI3K-Interconnection between Early and Late Signaling.Cells. 2022 Mar 10;11(6):953. doi: 10.3390/cells11060953. Cells. 2022. PMID: 35326404 Free PMC article.
-
Inhibition of Orai Channel Function Regulates Mas-Related G Protein-Coupled Receptor-Mediated Responses in Mast Cells.Front Immunol. 2022 Jan 20;12:803335. doi: 10.3389/fimmu.2021.803335. eCollection 2021. Front Immunol. 2022. PMID: 35126366 Free PMC article.
-
Identification of Gain and Loss of Function Missense Variants in MRGPRX2's Transmembrane and Intracellular Domains for Mast Cell Activation by Substance P.Int J Mol Sci. 2019 Oct 23;20(21):5247. doi: 10.3390/ijms20215247. Int J Mol Sci. 2019. PMID: 31652731 Free PMC article.
-
Recent advances in mast cell activation and regulation.F1000Res. 2020 Mar 19;9:F1000 Faculty Rev-196. doi: 10.12688/f1000research.22037.1. eCollection 2020. F1000Res. 2020. PMID: 32226609 Free PMC article. Review.
-
Murepavadin, a Small Molecule Host Defense Peptide Mimetic, Activates Mast Cells via MRGPRX2 and MrgprB2.Front Immunol. 2021 Jun 23;12:689410. doi: 10.3389/fimmu.2021.689410. eCollection 2021. Front Immunol. 2021. PMID: 34248979 Free PMC article.
References
-
- Laxminarayan R, Duse A, Wattal C, Zaidi AK, Wertheim HF, Sumpradit N, Vlieghe E, Hara GL, Gould IM, Goossens H, Greko C, So AD, Bigdeli M, Tomson G, Woodhouse W, Ombaka E, Peralta AQ, Qamar FN, Mir F, Kariuki S, Bhutta ZA, Coates A, Bergstrom R, Wright GD, Brown ED, and Cars O 2013. Antibiotic resistance-the need for global solutions. Lancet Infect Dis 13: 1057–1098. - PubMed
-
- Hancock RE, and Diamond G 2000. The role of cationic antimicrobial peptides in innate host defences. Trends Microbiol 8: 402–410. - PubMed
-
- Shai Y 2002. From innate immunity to de-novo designed antimicrobial peptides. Current pharmaceutical design 8: 715–725. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

