Uric acid activates aldose reductase and the polyol pathway for endogenous fructose and fat production causing development of fatty liver in rats
- PMID: 30651350
- PMCID: PMC6422088
- DOI: 10.1074/jbc.RA118.006158
Uric acid activates aldose reductase and the polyol pathway for endogenous fructose and fat production causing development of fatty liver in rats
Abstract
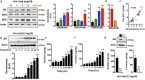
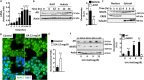
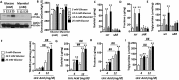
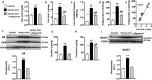
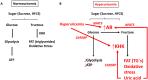
Dietary, fructose-containing sugars have been strongly associated with the development of nonalcoholic fatty liver disease (NAFLD). Recent studies suggest that fructose also can be produced via the polyol pathway in the liver, where it may induce hepatic fat accumulation. Moreover, fructose metabolism yields uric acid, which is highly associated with NAFLD. Here, using biochemical assays, reporter gene expression, and confocal fluorescence microscopy, we investigated whether uric acid regulates aldose reductase, a key enzyme in the polyol pathway. We evaluated whether soluble uric acid regulates aldose reductase expression both in cultured hepatocytes (HepG2 cells) and in the liver of hyperuricemic rats and whether this stimulation is associated with endogenous fructose production and fat accumulation. Uric acid dose-dependently stimulated aldose reductase expression in the HepG2 cells, and this stimulation was associated with endogenous fructose production and triglyceride accumulation. This stimulatory mechanism was mediated by uric acid-induced oxidative stress and stimulation of the transcription factor nuclear factor of activated T cells 5 (NFAT5). Uric acid also amplified the effects of elevated glucose levels to stimulate hepatocyte triglyceride accumulation. Hyperuricemic rats exhibited elevated hepatic aldose reductase expression, endogenous fructose accumulation, and fat buildup that was significantly reduced by co-administration of the xanthine oxidase inhibitor allopurinol. These results suggest that uric acid generated during fructose metabolism may act as a positive feedback mechanism that stimulates endogenous fructose production by stimulating aldose reductase in the polyol pathway. Our findings suggest an amplifying mechanism whereby soft drinks rich in glucose and fructose can induce NAFLD.
Keywords: aldose reductase; fatty acid; fructose; liver metabolism; metabolic syndrome; polyol pathway; sorbitol; uric acid.
© 2019 Sanchez-Lozada et al.
Conflict of interest statement
Some of the authors are members of Colorado Research Partners LLC, a start-up company developing inhibitors of fructose metabolism (M. A. L., A. A.-H., C. A. R.-J., R. J. J., and L. G. S.-L.). Dr. Johnson also has equity with a start-up company (XORT Therapeutics) developing novel xanthine oxidase inhibitors
Figures

References
-
- Abdelmalek M. F., Lazo M., Horska A., Bonekamp S., Lipkin E. W., Balasubramanyam A., Bantle J. P., Johnson R. J., Diehl A. M., Clark J. M., and Fatty Liver Subgroup of Look AHEAD Research Group (2012) Higher dietary fructose is associated with impaired hepatic adenosine triphosphate homeostasis in obese individuals with type 2 diabetes. Hepatology 56, 952–960 10.1016/j.jhep.2011.08.025,10.1002/hep.25741 - DOI - PMC - PubMed
-
- Abdelmalek M. F., Suzuki A., Guy C., Unalp-Arida A., Colvin R., Johnson R. J., Diehl A. M., and Nonalcoholic Steatohepatitis Clinical Research Network (2010) Increased fructose consumption is associated with fibrosis severity in patients with nonalcoholic fatty liver disease. Hepatology 51, 1961–1971 10.1002/hep.23535 - DOI - PMC - PubMed
-
- Jensen T., Abdelmalek M. F., Sullivan S., Nadeau K. J., Green M., Roncal C., Nakagawa T., Kuwabara M., Sato Y., Kang D. H., Tolan D. R., Sanchez-Lozada L. G., Rosen H. R., Lanaspa M. A., Diehl A. M., et al. (2018) Fructose and sugar: A major mediator of non-alcoholic fatty liver disease. J. Hepatol. 68, 1063–1075 10.1016/j.jhep.2018.01.019 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

