Equine Herpesvirus 1 Bridles T Lymphocytes To Reach Its Target Organs
- PMID: 30651370
- PMCID: PMC6430527
- DOI: 10.1128/JVI.02098-18
Equine Herpesvirus 1 Bridles T Lymphocytes To Reach Its Target Organs
Abstract
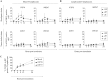
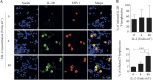
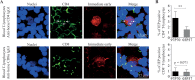
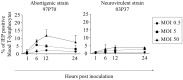
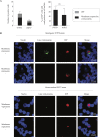
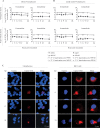
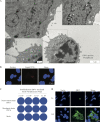
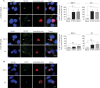
Equine herpesvirus 1 (EHV1) replicates in the respiratory epithelium and disseminates through the body via a cell-associated viremia in leukocytes, despite the presence of neutralizing antibodies. "Hijacked" leukocytes, previously identified as monocytic cells and T lymphocytes, transmit EHV1 to endothelial cells of the endometrium or central nervous system, causing reproductive (abortigenic variants) or neurological (neurological variants) disorders. In the present study, we questioned the potential route of EHV1 infection of T lymphocytes and how EHV1 misuses T lymphocytes as a vehicle to reach the endothelium of the target organs in the absence or presence of immune surveillance. Viral replication was evaluated in activated and quiescent primary T lymphocytes, and the results demonstrated increased infection of activated versus quiescent, CD4+ versus CD8+, and blood- versus lymph node-derived T cells. Moreover, primarily infected respiratory epithelial cells and circulating monocytic cells efficiently transferred virions to T lymphocytes in the presence of neutralizing antibodies. Albeit T-lymphocytes express all classes of viral proteins early in infection, the expression of viral glycoproteins on their cell surface was restricted. In addition, the release of viral progeny was hampered, resulting in the accumulation of viral nucleocapsids in the T cell nucleus. During contact of infected T lymphocytes with endothelial cells, a late viral protein(s) orchestrates T cell polarization and synapse formation, followed by anterograde dynein-mediated transport and transfer of viral progeny to the engaged cell. This represents a sophisticated but efficient immune evasion strategy to allow transfer of progeny virus from T lymphocytes to adjacent target cells. These results demonstrate that T lymphocytes are susceptible to EHV1 infection and that cell-cell contact transmits infectious virus to and from T lymphocytes.IMPORTANCE Equine herpesvirus 1 (EHV1) is an ancestral alphaherpesvirus that is related to herpes simplex virus 1 and causes respiratory, reproductive, and neurological disorders in Equidae. EHV1 is indisputably a master at exploiting leukocytes to reach its target organs, accordingly evading the host immunity. However, the role of T lymphocytes in cell-associated viremia remains poorly understood. Here we show that activated T lymphocytes efficiently become infected and support viral replication despite the presence of protective immunity. We demonstrate a restricted expression of viral proteins on the surfaces of infected T cells, which prevents immune recognition. In addition, we indicate a hampered release of progeny, which results in the accumulation of nucleocapsids in the T cell nucleus. Upon engagement with the target endothelium, late viral proteins orchestrate viral synapse formation and viral transfer to the contact cell. Our findings have significant implications for the understanding of EHV1 pathogenesis, which is essential for developing innovative therapies to prevent the devastating clinical symptoms of infection.
Keywords: Equidae; T lymphocytes; equine herpesvirus; immune evasion.
Copyright © 2019 Poelaert et al.
Figures



References
-
- Roizman B. 1996. Herpesviridae, p 2221–2230. In Fields BN, Knipe DM, Howley PM (ed), Fields virology, 3rd ed Lippincott-Raven Publishers; Philadelphia, PA.
-
- Allen G, Kydd J, Slater J, Smith K. 2004. Equid herpesvirus-1 (EHV-1) and -4 (EHV-4) infections, p 829–859. In Coetzer J, Tustin R (ed), Infectious diseases of livestock, 2nd ed Oxford Press; Cape Town, South Africa.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

