Evaluation of the Performance of the Cobas CT/NG Test for Use on the Cobas 6800/8800 Systems for Detection of Chlamydia trachomatis and Neisseria gonorrhoeae in Male and Female Urogenital Samples
- PMID: 30651389
- PMCID: PMC6440774
- DOI: 10.1128/JCM.01996-18
Evaluation of the Performance of the Cobas CT/NG Test for Use on the Cobas 6800/8800 Systems for Detection of Chlamydia trachomatis and Neisseria gonorrhoeae in Male and Female Urogenital Samples
Abstract
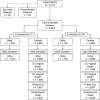
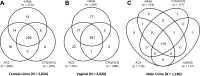
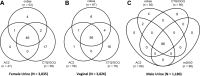
The clinical performance of the Cobas CT/NG assay on the Cobas 6800/8800 systems (Cobas) for the detection of Chlamydia trachomatis and Neisseria gonorrhoeae was established in a multisite, prospective collection study using male and female urogenital specimens; supportive data from archived specimens were also included. The results obtained with the Cobas assay were compared with the patient infected status derived from a combination of U.S. Food and Drug Administration-approved nucleic acid amplification tests to determine the sensitivity and specificity of detection from each sample type. The sensitivity of Cobas for the detection of C. trachomatis in female specimens was 95.6% (95% confidence interval [CI], 92.4% to 97.4%) for urine; 98.6% (95% CI, 95.2% to 99.6%) and 99.2% (95% CI, 95.4% to 99.9%) for clinician- and self-collected vaginal swab specimens, respectively; 93.3% (95% CI, 89.6% to 95.7%) for endocervical swabs; and 92.5% (95% CI, 88.7% to 95.1%) for cervical swab samples in PreservCyt. The specificity for the detection of C. trachomatis was ≥98.8% for all female sample types. Sensitivity and specificity estimates of Cobas for the detection of C. trachomatis in male urine samples were 100% (96.8% to 100.0%) and 99.7% (95% CI, 99.2% to 99.9%), respectively. The sensitivity of Cobas for the detection of N. gonorrhoeae in female specimens was 94.8% (95% CI, 89.6% to 97.4%) for urine; 100.0% (95% CI, 87.9% to 100.0%) and 100.0% (95% CI, 87.9% to 100.0%) for clinician- and self-collected vaginal swab specimens, respectively; 97.0% (95% CI, 91.5% to 99.0%) for endocervical swabs; and 96.6% (95% CI, 90.6% to 98.8%) for cervical samples in PreservCyt; the specificity for all female sample types was >99.0%. The sensitivity and specificity of Cobas for detecting N. gonorrhoeae in male urine were 100.0% (95% CI, 95.8% to 100.0%) and 99.5% (95% CI, 98.8% to 99.8%), respectively. Fully automated assays help fill the clinical need for a sensitive, high-throughput screening tool to aid public health efforts to control C. trachomatis and N. gonorrhoeae infections.
Keywords: Chlamydia trachomatis; Cobas CT/NG assay; Neisseria gonorrhoeae; PCR; genital infection; molecular diagnostics; nucleic acid amplification test; sexually transmitted infection.
Copyright © 2019 Van Der Pol et al.
Figures
References
-
- Newman L, Rowley J, Vander Hoorn S, Wijesooriya NS, Unemo M, Low N, Stevens G, Gottlieb S, Kiarie J, Temmerman M. 2015. Global estimates of the prevalence and incidence of four curable sexually transmitted infections in 2012 based on systematic review and global reporting. PLoS One 10:e0143304. doi: 10.1371/journal.pone.0143304. - DOI - PMC - PubMed
-
- Centers for Disease Control and Prevention. 2018. Sexually transmitted disease surveillance 2017. U.S. Department of Health and Human Services, Atlanta, GA.

