Diagnosing Invasive Pulmonary Aspergillosis in Hematology Patients: a Retrospective Multicenter Evaluation of a Novel Lateral Flow Device
- PMID: 30651395
- PMCID: PMC6440777
- DOI: 10.1128/JCM.01913-18
Diagnosing Invasive Pulmonary Aspergillosis in Hematology Patients: a Retrospective Multicenter Evaluation of a Novel Lateral Flow Device
Abstract
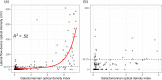
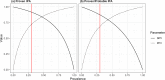
Invasive pulmonary aspergillosis (IPA) is a potentially lethal infection in patients with hematological diseases or following allogeneic stem cell transplantation. Early diagnosis is essential, as delayed treatment results in increased mortality. Recently, a lateral flow device (LFD) for the diagnosis of IPA was CE marked and made commercially available by OLM Diagnostics. We retrospectively analyzed bronchoalveolar lavage fluid (BALf) collected from adult hematology patients from 4 centers in The Netherlands and Belgium. Galactomannan was retested in all samples. All samples were applied to an LFD and read out visually by two independent researchers blinded to the diagnosis of the patient. All samples were also read out using a digital reader. We included 11 patients with proven IPA, 68 patients with probable IPA, 44 patients with possible IPA, and 124 patients with no signs of IPA (controls). In cases of proven IPA versus controls, sensitivity and specificity were 0.82 and 0.86 for visual readout and 0.82 and 0.96 for digital readout, respectively. When comparing patients with proven and probable IPA as cases versus controls, sensitivity and specificity were found to be 0.71 and 0.86, respectively. When excluding serum and BALf galactomannan as mycological criteria from the 2008 European Organization for Research and Treatment of Cancer Invasive Fungal Infections Cooperative Group (EORTC)/Mycoses Study Group of the National Institute of Allergy and Infectious Diseases (MSG) consensus definitions, the LFD was less specific than galactomannan when comparing subjects with proven and probable IPA to controls (0.86 versus 0.96; P = 0.005) but had similar sensitivity (0.76 versus 0.85; P = 0.18). In conclusions, in this large study of the CE-marked LFD in BALf from hematology patients, the LFD had a good performance for the diagnosis of IPA.
Keywords: diagnosis; galactomannan; invasive aspergillosis; lateral flow device.
Copyright © 2019 American Society for Microbiology.
Figures


Similar articles
-
Lateral flow assays for diagnosing invasive pulmonary aspergillosis in adult hematology patients: A comparative multicenter study.Med Mycol. 2020 Jun 1;58(4):444-452. doi: 10.1093/mmy/myz079. Med Mycol. 2020. PMID: 31290552
-
Detection of invasive pulmonary aspergillosis in haematological malignancy patients by using lateral-flow technology.J Vis Exp. 2012 Mar 22;(61):3721. doi: 10.3791/3721. J Vis Exp. 2012. PMID: 22473419 Free PMC article.
-
Aspergillus-Specific Lateral-Flow Device and Real-Time PCR Testing of Bronchoalveolar Lavage Fluid: a Combination Biomarker Approach for Clinical Diagnosis of Invasive Pulmonary Aspergillosis.J Clin Microbiol. 2015 Jul;53(7):2103-8. doi: 10.1128/JCM.00110-15. Epub 2015 Apr 22. J Clin Microbiol. 2015. PMID: 25903568 Free PMC article.
-
The performance of galactomannan in combination with 1,3-β-D-glucan or aspergillus-lateral flow device for the diagnosis of invasive aspergillosis: Evidences from 13 studies.Diagn Microbiol Infect Dis. 2019 Jan;93(1):44-53. doi: 10.1016/j.diagmicrobio.2018.08.005. Epub 2018 Aug 18. Diagn Microbiol Infect Dis. 2019. PMID: 30279025
-
Invasive Pulmonary Aspergillosis.Semin Respir Crit Care Med. 2020 Feb;41(1):80-98. doi: 10.1055/s-0039-3401990. Epub 2020 Jan 30. Semin Respir Crit Care Med. 2020. PMID: 32000286 Review.
Cited by
-
Identification of Mycoses in Developing Countries.J Fungi (Basel). 2019 Sep 29;5(4):90. doi: 10.3390/jof5040090. J Fungi (Basel). 2019. PMID: 31569472 Free PMC article. Review.
-
Monoclonal Antibodies and Invasive Aspergillosis: Diagnostic and Therapeutic Perspectives.Int J Mol Sci. 2022 May 16;23(10):5563. doi: 10.3390/ijms23105563. Int J Mol Sci. 2022. PMID: 35628374 Free PMC article. Review.
-
Aspergillus fumigatus and Its Allergenic Ribotoxin Asp f I: Old Enemies but New Opportunities for Urine-Based Detection of Invasive Pulmonary Aspergillosis Using Lateral-Flow Technology.J Fungi (Basel). 2020 Dec 31;7(1):19. doi: 10.3390/jof7010019. J Fungi (Basel). 2020. PMID: 33396482 Free PMC article.
-
Invasive Pulmonary Aspergillosis.J Fungi (Basel). 2023 Jan 17;9(2):131. doi: 10.3390/jof9020131. J Fungi (Basel). 2023. PMID: 36836246 Free PMC article. Review.
-
More than meets the eye: Nocardia farcinica, Candida dubliniensis and Aspergillus spp. co-infection in a patient with multiple myeloma treated with multiple treatment regimens.BMC Infect Dis. 2025 Feb 3;25(1):156. doi: 10.1186/s12879-024-10387-z. BMC Infect Dis. 2025. PMID: 39901105 Free PMC article.
References
-
- Leeflang MMG, Debets-Ossenkopp YJ, Visser CE, Scholten RJPM, Hooft L, Bijlmer HA, Reitsma JB, Bossuyt PMM, Vandenbroucke-Grauls CM. 2008. Galactomannan detection for invasive aspergillosis in immunocompromized patients. Cochrane Database Syst Rev 2008:CD007394. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

