The HisCl1 histamine receptor acts in photoreceptors to synchronize Drosophila behavioral rhythms with light-dark cycles
- PMID: 30651542
- PMCID: PMC6335465
- DOI: 10.1038/s41467-018-08116-7
The HisCl1 histamine receptor acts in photoreceptors to synchronize Drosophila behavioral rhythms with light-dark cycles
Abstract
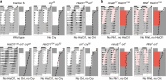
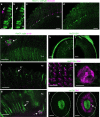
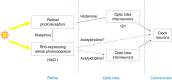
In Drosophila, the clock that controls rest-activity rhythms synchronizes with light-dark cycles through either the blue-light sensitive cryptochrome (Cry) located in most clock neurons, or rhodopsin-expressing histaminergic photoreceptors. Here we show that, in the absence of Cry, each of the two histamine receptors Ort and HisCl1 contribute to entrain the clock whereas no entrainment occurs in the absence of the two receptors. In contrast to Ort, HisCl1 does not restore entrainment when expressed in the optic lobe interneurons. Indeed, HisCl1 is expressed in wild-type photoreceptors and entrainment is strongly impaired in flies with photoreceptors mutant for HisCl1. Rescuing HisCl1 expression in the Rh6-expressing photoreceptors restores entrainment but it does not in other photoreceptors, which send histaminergic inputs to Rh6-expressing photoreceptors. Our results thus show that Rh6-expressing neurons contribute to circadian entrainment as both photoreceptors and interneurons, recalling the dual function of melanopsin-expressing ganglion cells in the mammalian retina.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

