Epigenetic regulation in AKI and kidney repair: mechanisms and therapeutic implications
- PMID: 30651611
- PMCID: PMC7866490
- DOI: 10.1038/s41581-018-0103-6
Epigenetic regulation in AKI and kidney repair: mechanisms and therapeutic implications
Abstract
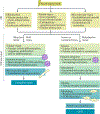
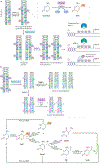
Acute kidney injury (AKI) is a major public health concern associated with high morbidity and mortality. Despite decades of research, the pathogenesis of AKI remains incompletely understood and effective therapies are lacking. An increasing body of evidence suggests a role for epigenetic regulation in the process of AKI and kidney repair, involving remarkable changes in histone modifications, DNA methylation and the expression of various non-coding RNAs. For instance, increases in levels of histone acetylation seem to protect kidneys from AKI and promote kidney repair. AKI is also associated with changes in genome-wide and gene-specific DNA methylation; however, the role and regulation of DNA methylation in kidney injury and repair remains largely elusive. MicroRNAs have been studied quite extensively in AKI, and a plethora of specific microRNAs have been implicated in the pathogenesis of AKI. Emerging research suggests potential for microRNAs as novel diagnostic biomarkers of AKI. Further investigation into these epigenetic mechanisms will not only generate novel insights into the mechanisms of AKI and kidney repair but also might lead to new strategies for the diagnosis and therapy of this disease.
Figures


References
-
- Lameire NH et al. Acute kidney injury: an increasing global concern. Lancet 382, 170–179 (2013). - PubMed
-
- Mehta RL et al. Recognition and management of acute kidney injury in the International Society of Nephrology 0by25 Global Snapshot: a multinational cross-sectional study. Lancet 387, 2017–2025 (2016). - PubMed
-
- Mehta RL et al. International Society of Nephrology’s 0by25 initiative for acute kidney injury (zero preventable deaths by 2025): a human rights case for nephrology. Lancet 385, 2616–2643 (2015). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

