Targeting FLT3 mutations in AML: review of current knowledge and evidence
- PMID: 30651634
- PMCID: PMC6365380
- DOI: 10.1038/s41375-018-0357-9
Targeting FLT3 mutations in AML: review of current knowledge and evidence
Abstract
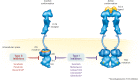
Genomic investigations of acute myeloid leukemia (AML) have demonstrated that several genes are recurrently mutated, leading to new genomic classifications, predictive biomarkers, and new therapeutic targets. Mutations of the FMS-like tyrosine kinase 3 (FLT3) gene occur in approximately 30% of all AML cases, with the internal tandem duplication (ITD) representing the most common type of FLT3 mutation (FLT3-ITD; approximately 25% of all AML cases). FLT3-ITD is a common driver mutation that presents with a high leukemic burden and confers a poor prognosis in patients with AML. The prognostic value of a FLT3 mutation in the tyrosine kinase domain (FLT3-TKD), which has a lower incidence in AML (approximately 7-10% of all cases), is uncertain. Accumulating evidence demonstrates that FLT3 mutational status evolves throughout the disease continuum. This so-called clonal evolution, together with the identification of FLT3-ITD as a negative prognostic marker, serves to highlight the importance of FLT3-ITD testing at diagnosis and again at relapse. Earlier identification of FLT3 mutations will help provide a better understanding of the patient's disease and enable targeted treatment that may help patients achieve longer and more durable remissions. First-generation FLT3 inhibitors developed for clinical use are broad-spectrum, multikinase inhibitors; however, next-generation FLT3 inhibitors are more specific, more potent, and have fewer toxicities associated with off-target effects. Primary and secondary acquired resistance to FLT3 inhibitors remains a challenge and provides a rationale for combining FLT3 inhibitors with other therapies, both conventional and investigational. This review focuses on the pathological and prognostic role of FLT3 mutations in AML, clinical classification of the disease, recent progress with next-generation FLT3 inhibitors, and mechanisms of resistance to FLT3 inhibitors.
Conflict of interest statement
ND has received research funding from Daiichi Sankyo, Bristol-Myers Squibb, Pfizer, Karyopharm, Sevier, Genentech, and ImmunoGen and has served in a consulting or advisory role for Daiichi Sankyo, Bristol-Myers Squibb, Pfizer, Novartis, Celgene, AbbVie, and Agios. RFS has received honoraria from Daiichi Sankyo, Novartis, Pfizer, Janssen, and Arog; has served in a consulting or advisory role for Daiichi Sankyo, Novartis, and Pfizer; and has received research funding from Pfizer, AstraZeneca, PharmaMar, and Novartis. NHR has received honoraria from and has served in an advisory role for Jazz, Teva, Pfizer, and Daiichi Sankyo. MJL has received honoraria from Daiichi Sankyo, Novartis, and Agios; has served in a consulting or advisory role for Daiichi Sankyo, Novartis, and Agios; and has received research funding from Astellas and Novartis.
Figures
References
-
- Döhner H, Weisdorf DJ, Bloomfield CD. Acute myeloid leukemia. N Engl J Med. 2015;373:1136–52. - PubMed
-
- O’Donnell MR, Tallman MS, Abboud CN, Altman JK, Appelbaum FR, Arber DA, et al. Acute myeloid leukemia, version 3.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2017;15:926–57. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

