Human blood vessel organoids as a model of diabetic vasculopathy
- PMID: 30651639
- PMCID: PMC7116578
- DOI: 10.1038/s41586-018-0858-8
Human blood vessel organoids as a model of diabetic vasculopathy
Abstract
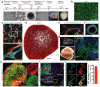
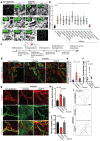
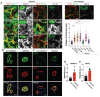
The increasing prevalence of diabetes has resulted in a global epidemic1. Diabetes is a major cause of blindness, kidney failure, heart attacks, stroke and amputation of lower limbs. These are often caused by changes in blood vessels, such as the expansion of the basement membrane and a loss of vascular cells2-4. Diabetes also impairs the functions of endothelial cells5 and disturbs the communication between endothelial cells and pericytes6. How dysfunction of endothelial cells and/or pericytes leads to diabetic vasculopathy remains largely unknown. Here we report the development of self-organizing three-dimensional human blood vessel organoids from pluripotent stem cells. These human blood vessel organoids contain endothelial cells and pericytes that self-assemble into capillary networks that are enveloped by a basement membrane. Human blood vessel organoids transplanted into mice form a stable, perfused vascular tree, including arteries, arterioles and venules. Exposure of blood vessel organoids to hyperglycaemia and inflammatory cytokines in vitro induces thickening of the vascular basement membrane. Human blood vessels, exposed in vivo to a diabetic milieu in mice, also mimic the microvascular changes found in patients with diabetes. DLL4 and NOTCH3 were identified as key drivers of diabetic vasculopathy in human blood vessels. Therefore, organoids derived from human stem cells faithfully recapitulate the structure and function of human blood vessels and are amenable systems for modelling and identifying the regulators of diabetic vasculopathy, a disease that affects hundreds of millions of patients worldwide.
Conflict of interest statement
Figures
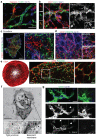



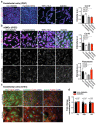


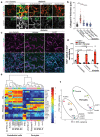

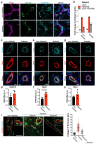

Comment in
-
A new organoid model to study diabetic vasculopathy.Nat Rev Cardiol. 2019 Mar;16(3):131. doi: 10.1038/s41569-019-0165-6. Nat Rev Cardiol. 2019. PMID: 30710151 No abstract available.
-
Modelling diabetic vasculopathy with human vessel organoids.Nat Rev Nephrol. 2019 May;15(5):258-260. doi: 10.1038/s41581-019-0125-8. Nat Rev Nephrol. 2019. PMID: 30778152 Free PMC article.
-
Vascular Organoids: Are We Entering a New Area of Cardiometabolic Research?Cell Metab. 2019 Apr 2;29(4):792-794. doi: 10.1016/j.cmet.2019.03.008. Cell Metab. 2019. PMID: 30943391
References
-
- World Health Organization. Global Report on Diabetes. 2016 ISBN 978.
-
- Roy S, Ha J, Trudeau K, Beglova E. Vascular Basement Membrane Thickening in Diabetic Retinopathy. Curr Eye Res. 2010;35:1045–1056. - PubMed
-
- Fowler MJ. Microvascular and macrovascular complications of diabetes. Clin Diabetes. 2011;29:116–122.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

